Tripartite-motif Protein Family (TRIM)
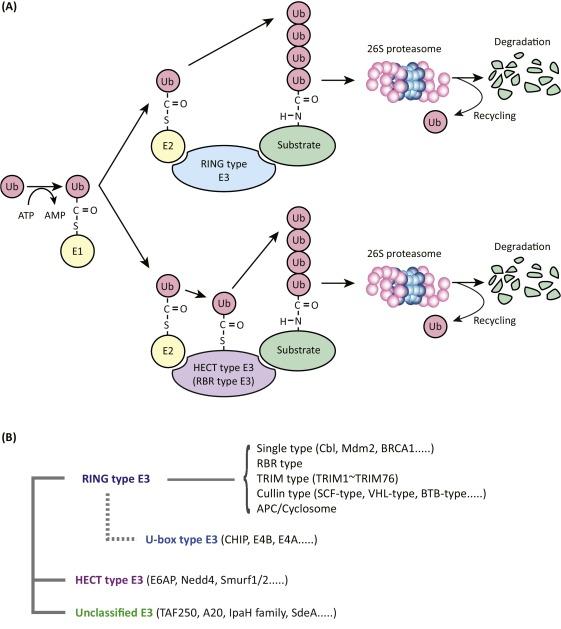
TRIM is composed of more than 80 proteins with E3 ubiquitin ligase activity. According to the different structures of TRIM proteins, they are divided into 11 subfamilies (C-I-C-Ⅺ), of which the C-Ⅳ subfamily has the most members, including 33 TRIMs protein, other family proteins are less. Due to their complex properties, TRIM proteins are involved in the regulation of many cellular pathways, including cell proliferation, promotion or inhibition of cell transformation to cancer, cell metabolism, autophagy, etc. TRIM11 is one of the important members of the TRIM C-IV family, located on human chromosome 1q42.13, contains an open reading frame and 6 exons, is about 2 715 bp long, and encodes a 55 kd protein. It is an RBCC motif at the n-terminal, which contains a ring finger domain, 1 or 2 B-box motifs and a helical region, and a PRY-SPRY (PS) motif at the c-terminal. Due to its ring finger domain, it can act as an E3 ubiquitin ligase to regulate post-translational modifications of proteins, such as phosphorylation and ubiquitination, which in turn affect the degradation of some proteins and the body’s immune response. Dysregulated TRIM11 activates related signals pathways, which further affect the phenotype of tumor cells and are closely related to tumorigenesis and tumor development.
Relationship Between TRIM11 and Tumor
Figure 1. TRIM Family Proteins: Roles in Autophagy, Immunity, and Carcinogenesis.
As an oncogene, TRIM11 can lead to poor prognosis of tumor patients by increasing expression, targeting related signaling pathways, promoting tumor proliferation, anti-apoptosis, invasion and metastasis, and chemotherapy resistance. Proliferation and apoptosis Sustained proliferation and anti-apoptosis are the basic conditions for the occurrence and development of tumors. TRIM11 overexpression plays an important role in tumor proliferation and apoptosis. A study explored the effect of TRIM11 on the viability and apoptosis of human breast cancer cell lines MCF-7 and MDA-MB-231 cells, and found that the downregulation of TRIM11 significantly reduced the proliferation rate of MCF-7 and MDA-MB-231 cells, while TRIM11 The cell proliferation rate was significantly increased when overexpressed, and TRIM11 was further down-regulated to explore the anti-apoptotic mechanism of breast cancer cells. It was found that when TRIM11 was down-regulated, the expression of apoptosis-related genes PTEN, p53 and Bax increased significantly, while after TRIM11 was up-regulated , the expressions of PTEN, p53 and Bax decreased significantly.
Invasion and Metastasis
Members of the TRIM family play a vital role in tumor invasion and metastasis. Studies have found that the expression of TRIM11 protein is positively correlated with postoperative metastasis and recurrence of tumors, and the expression of TRIM11 in stage Ⅲ/Ⅳ hepatocellular carcinoma is significantly higher than that in stage Ⅰ/Ⅳ. In stage II hepatocellular carcinoma, TRIM11 expression was positively correlated with serum alpha-fetoprotein level. In addition, endometriosis is an early event of tumor metastasis, through which cancer cells acquire aggressive mesenchymal properties, resulting in decreased adhesion and cell polarity, enhanced mobility and invasiveness, and ultimately the migration of primary tumor tissue to adjacent tissues. even distant metastasis.
Drug Resistance
Chemotherapy resistance is a major cause of cancer treatment failure. Members of the TRIM family also play an important role in chemotherapy failure. Studies have found that TRIM11 promotes drug resistance in nasopharyngeal carcinoma by regulating the Daple-Dvl-β-catenin-ABCC9 signaling pathway. It was also found that TRIM11 was increased in cisplatin- and paclitaxel-resistant breast cancer tissues, and downregulation of TRIM11 enhanced the pro-apoptotic effect of chemotherapeutic drugs. In addition, gemcitabine is a first-line chemotherapy drug for pancreatic cancer, but its efficacy is limited by drug resistance. In the study, it was found that the expression of TRIM11 was higher in pancreatic ductal adenocarcinoma (pancreatic ductal adenocarcinomas, PDAC) cells and tissues, and the overexpression of TRIM11 promoted the proliferation of PDAC cells in vitro and tumor growth in vivo. The reduction of TRIM11 was related to the increase of TAX1BP1 expression. Mechanistically, TRIM11 promoted gemcitabine resistance and inhibited ferritin phagocytosis through UBE2N-TAX1BP1 signaling pathway. Therefore, TRIM11 is a key regulator of the TAX1BP1 signaling pathway and plays an important role in the process of ferritin phagocytosis and gemcitabine resistance in PDAC.