AMOT family, also known as the Motin family, has three members: angiomotin (AMOT), angiomotin-like protein 1 (AMOTL1), and angiomotin-like protein 2 (AMOTL2). There are two shear isomers of AMOT, AMOT-P130 and AMOT-P80. AMOT was first discovered and named in 2001. Existing studies have shown that AMOTs play a central role in tight junctions, cell migration, angiogenesis, and virus release. AMOTs are also involved in the occurrence and progression of cancer, and play different functions in different cancers. AMOTs exhibit a tumor-promoting effect in some cancer cells, and a tumor-suppressing effect in another part of cancer cells. The molecular mechanism of how AMOTs regulate their own functions and cancer progression still needs to be explored.
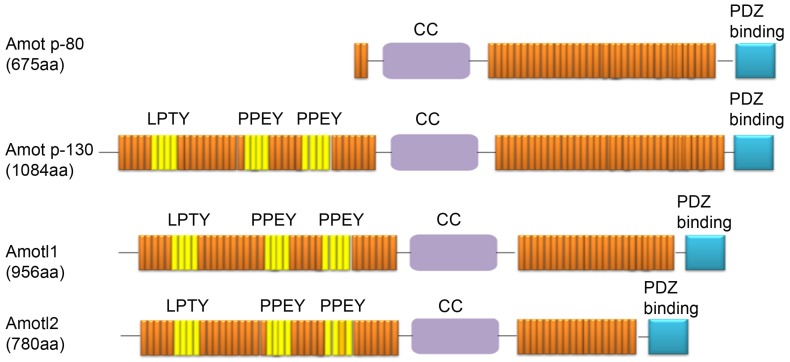
AMOTs family has similar protein structures. AMOT-P130, AMOTL1, and AMOTL2 share a filamentous actin (F-actin) binding motif and three L/PPxY motifs, except AMOT-P80, which is deleted at the N-terminus. AMOTs share a colied-coil (CC) domain at the C-terminus and a discoid homology region (PDZ) binding motif at the terminal end. AMOT-P130 and AMOT-P80 also have a unique angiostatin binding domain, which is missing in AMOTL1 and AMOTL2.
The L/PPxY motif is crucial to the interaction of proteins in organisms, and it mainly recognizes and binds to the WW domain. Their modular interactions are involved in many important processes such as signal transduction in cells, regulation of protein ubiquitination, and protein spatial localization. AMOTs regulate the HIPPO-YAP signaling pathway and virus release process through the L/PPxY motif. The F-actin binding motif is a small amino acid sequence located at the N-terminus of AMOTs. It is mainly responsible for the combination of AMOT and F-actin and the positioning of AMOT in the cytoskeleton. It is very important for cell proliferation and cell shape regulation controlled by AMOTs through the transcriptional coactivator YAP. The CC domain usually consists of two or more α-helical twisted windings and is a common domain involved in protein folding and protein interaction. The CC domain can also regulate the homo-oligomerization of the protein itself and the hetero-oligomerization between different proteins. All members of the AMOT family have been reported to undergo oligomerization, which is essential for their biological function and stability. In addition, PDZ-binding motifs generally only contain 4-5 amino acid residues and often exist on various polar proteins and tight junction resident proteins, which are involved in regulating the localization and cell migration of AMOT. The angiostatin binding domain is a hydrophobic domain present in AMOT-P130 and AMOT-P80, and some studies have shown that it can affect the cell membrane localization of AMOT. Angiostatin inhibits angiogenesis by binding to the angiostatin-binding domain of AMOT.
Figure 1. Domain structures of proteins from the Amot family.
Expression Localization of AMOTs
In different tissues and cells, AMOTs have different expression and localization. Although they also have different expression levels and variable spatial localization in the same tissue and cell. AMOTs are expressed in almost all human tissues, but at different levels. The transcript level of AMOT was highest in male tissues, followed by proximal alimentary canal tissues, and lowest in retina. AMOTL1 was highly expressed in muscle tissue and proximal digestive tract tissue. Connective and soft tissues contain high amounts of AMOTL2, but are barely detectable in the retina. When detecting the localization of endogenous AMOTs in different cell lines, it was found that in most endothelial cells, AMOT-P130, AMOT-P80 and AMOTL1 were mainly localized in the cytoskeleton, while AMOTL2 was localized in the tight junction of cells. In some epithelial cells, AMOTs can co-localize with F-actin in tight junctions of cells. In addition, AMOT and AMOTL1 can also function in the cytoplasm and nucleus.
Cellular Functions of AMOTs
AMOTs Regulate Angiogenesis
Angiogenesis is the generation of new blood vessels from existing blood vessels, involving a series of complex cell biological processes, including cell proliferation, migration, assembly, and tube formation. AMOT was identified as a direct binding protein of the angiogenesis inhibitor-angiostatin, which promotes endothelial cell migration and tube formation. AMOT-P130 and AMOT-P80 have been reported to play different roles in angiogenesis. AMOT-P80 primarily stimulates endothelial cell migration, whereas AMOT-P130 is primarily involved in vascular stabilization and maturation. Although the amino acid sequences of AMOTL1 and AMOTL2 do not have an angiostatin-binding domain like AMOT, their role in angiogenesis has been confirmed. AMOTL1 was identified as a novel component of the N-cadherin complex critical for vascular remodeling during angiogenesis. And AMOTL2 positively regulates the polarity, migration and proliferation of endothelial cells in angiogenesis through MAPK/ERK1/2 signaling. In addition, AMOTs is a part of endothelial integrin adhesion body, which helps to maintain the normal transmission of force in vasodilation, and its absence may inhibit developmental angiogenesis and tumor angiogenesis.
AMOTs Regulate the HIPPO-YAP Signaling Pathway
The HIPPO signaling pathway was first discovered in Drosophila and is highly conserved in mammals. The HIPPO-YAP pathway plays an important regulatory role in cell proliferation, organ development, cell apoptosis, and tissue regeneration. AMOTs regulate the HIPPO-YAP signaling pathway in many ways. First, AMOT-P130 can directly bind YAP1 and promote or inhibit the activity of YAP1. On the one hand, AMOT-P130 can bind YAP1 in both phosphorylated and non-phosphorylated states, causing YAP1 to be blocked in the cytoplasm. AMOT-P130 can also recruit YAP1 and E3 ubiquitin ligase AIP4 into a complex, which promotes the stability of AMOT-P130 and degrades YAP1 by increasing the enzymatic activity of AIP4. On the other hand, AMOT-P130 can also bind to YAP1 in the nucleus to promote the nuclear localization of YAP1 and the activation of downstream target genes. These opposite results may depend on the post-translational modification type and status of AMOT-P130.
AMOTs Regulate Wnt/β-catenin Signaling Pathway
The Wnt/β-catenin signaling pathway is one of the most important signaling pathways in cells, and its dysregulation is closely related to the occurrence and development of various cancers. Normal cytoplasmic-nuclear shuttling of the transcription factor β-catenin is critical for activation of this pathway. In mammalian cells, AMOTs can attenuate Wnt/β-catenin signal transduction, and the inhibitory effect of AMOTL2 is the most obvious. AMOTL2 can directly bind to β-catenin in circulating endosomes, block β-catenin in the cytoplasm, and inhibit its nuclear translocation and transcriptional activity. In addition, AXIN1, as a key scaffolding protein of the β-catenin degradation complex, can be jointly targeted to the proteasome for degradation by poly ADP ribosyltransferases Tankyrase (TNKS1 and TNKS2) and E3 ubiquitin ligase RNF146, which blocks the degradation of β-catenin. β-catenin degradation and stimulate Wnt/β-catenin signaling. AMOT-P130 competes with AXIN1 to bind TNKS1/2 through the N-terminal domain, thereby reducing the stability of β-catenin protein and inactivating Wnt/β-catenin signaling.
AMOTs Regulate Embryonic Development
The development of human embryo requires necessary signals from extra-embryonic tissues such as placenta, and the formation of placenta provides an important guarantee for the normal development of embryos during pregnancy. AMOT and AMOTL2 play different roles in embryonic development. Among them, AMOT-P130 and AMOT-P80 are expressed in the placenta as early as the 5th week of gestation, and the expression level increases significantly after the 10th week. In particular, AMOT-P80 can regulate the migration of trophoblast cells in the placenta. In addition, the study found that AMOT also plays a key role in the formation of new blood vessels during mouse and zebrafish embryonic development. There is evidence that AMOT is differentially expressed in two cell lineages arising in the early embryo, the inner cell mass (ICM) and trophectoderm (TE), and can prevent inappropriate cell fate programming in the multipotent ICM.
AMOTs regulate cell junctions Cell junctions are the connection of cells in tissues to each other through cell membranes, which is very important for the regulation of tissue homeostasis. In epithelial and endothelial cells, these cell-cell junctions regulate cell proliferation and cell migration processes through adhesion junctions (AJs) and tight junctions (TJs). AMOTs exist at the TJ of epithelial cells and form a complex with the Rho GTPase activating protein RICH1 to maintain TJ stability. AMOT colocalizes with ZO-1 at cell-cell contacts in endothelial cells in vitro and plays a role in the assembly of cell junctions. In addition, AMOTL2 directly binds the polarity protein Par3 via a PDZ-binding motif and coordinates and transduces signals from junctional and polar components as part of an adherens junction-associated signaling complex. AMOTL1 is a key regulator of junctional stability of dorsal aortic stem cells in zebrafish embryos.
AMOTs regulate virus release process Many literatures have reported the important role of AMOTs in virus release. AMOT-P130 can bind to the WW domain of NEDD4L through the L/PPxY motif, thereby promoting the release of enveloped virus HIV-1. In the absence of AMOT-P130, the HIV-1 particle envelope process is blocked and budding is inhibited. However, overexpression of AMOT-P80 failed to stimulate viral particle release. Still other viruses use the L/PPxY motif to mediate the host’s endosomal sorting complex (ESCRT) pathway for budding. However, PIV5M lacks the L/PPxY motif, and requires AMOTL1 to indirectly connect PIV5M and NEDD4 protein through its own C-terminal region and the L/PPxY motif, and then use the ESCRT pathway to participate in the regulation of the release of paramyxovirus particles. AMOT and AMOTL2 cannot bind PIV5M. In addition, the transmission of filoviruses is also regulated by AMOT. Ebola virus (EBOV) and Marburg virus (MARV) control virus particle assembly and budding through the VP40 matrix protein. Ectopically expressed YAP/TAZ can bind VP40 and inhibit virus particle release, but this inhibitory effect can be alleviated by AMOT-P130 through competitive binding.