The primary function of atrial natriuretic peptide (ANP) includes regulation of water and salt metabolism together with vascular volume which helps control blood pressure stability. Additionally, ANP manages endothelial cell permeability and counters the adrenal aldosterone system which allows it to control growth metabolism and influence myocardial hypertrophy and fibrosis regulation. Recent research shows that ANP and its receptors are present in several immune cells and organs where they perform immune regulation functions and play roles in allergic asthma development as well as pancreatitis and cancer progression. The article examines ANP structure and function along with its receptors’ immune regulation to advance research on immunological roles.
ANP Family
Natriuretic peptides represent a hormone family with structural similarities yet distinct encoding genes. The ANP family consists of three members which are brain natriuretic peptide (BNP), C-type natriuretic peptide (CNP), and ANP. Chromosome 1p36.2 houses the human ANP gene which produces approximately 28 amino acids and releases ANP primarily when atrial cells face tension stimulation. In atrial myocytes ANP is present as precursor molecules known as pro-ANP. Pro-ANP gets broken down into the nonfunctional NT-pro-ANP and the functional ANP which then move into the bloodstream through exocytosis to respond to atrial stretching due to systemic circulation overload. The secretion of ANP depends not only on mechanical stretching but also on multiple extracellular signals that include angiotensin along with catecholamines and vasopressin. Enkephalinase or neutral endopeptidase enzymes break down the inner ring structure of ANP. ANP can leave the bloodstream by attaching to the NPR-C receptor besides being broken down by enzymes. ANP mainly exerts its biological effects through two receptors, natriuretic receptor-A (NPR-A) and NPR-C, located on the plasma membrane of target cells.
ANP Receptors
The human NPR-A gene can be found at chromosomes 1q21-22 and measures approximately 16,000 base pairs and this gene product includes three distinct domains namely guanylate cyclase (GC) domain, extracellular natriuretic peptide binding domain and intracellular kinase domain. The cardiovascular system as well as kidneys, adrenal glands, terminal ileum, aorta and lung tissues all express it at high levels. The NPR-C gene occupies the chromosome region 5p14-p13 while being approximately 65,000 in size and it encodes a protein sequence of 540 amino acids. Its extracellular domain shares 30% identity with NPR-A while its intracellular domain comprises 37 amino acids without guanylate cyclase activity. The NPR-C receptor appears throughout the atria, mesentery, lungs, kidneys and vascular system while it is found abundantly in the cells lining blood vessels. Two NPR-C molecules form a disulfide linkage through their C-terminal cysteine residues C428 and C431 which results in dimers.
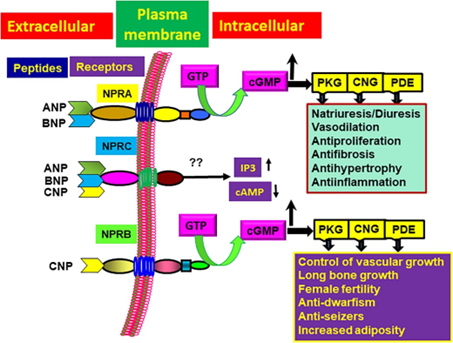
Figure 1. Ligand-binding specificity of different NPs (ANP, BNP, and CNP) with their cognate receptors (NPRA, NPRB, NPRC) and intracellular signaling mechanisms leading to the physiological responses.
NPR-A is the main receptor of ANP. NPR-A creates cGMP (CyclicGMP) and initiates effector molecule activation which produces a variety of physiological and pathological responses including control of cell growth proliferation and apoptosis alongside inflammatory processes. High ANP levels result in decreased NPRRA expression which diminishes the binding affinity between ANP and NPRRA according to studies. NPR-C binds strongly to ANP to enhance cAMP expression through modulation of adenylate cyclase activity. The activation of phospholipase C (PLC) by the substance results in higher levels of both inositol trisphosphate (IP3) and diacylglycerol (DAG). NPR-C removes natriuretic peptides from blood circulation and extracellular spaces by internalizing and breaking them down through receptor-mediated mechanisms making it known as a clearance receptor.
Physiological Functions of ANP
ANP mainly affects kidneys, blood vessels and adrenal glands. The physiological effects of ANP consist of natriuresis and diuresis along with vasodilation and the inhibition of renin and antidiuretic hormone and aldosterone release which leads to a reduction in total peripheral vascular resistance. The substance regulates endocrine functions and demonstrates growth inhibition in cells. The compound shows anti-proliferative action against astrocytes, endothelial cells, cardiac fibroblasts and multiple other cell types. ANP and its receptors show expression within lung tissue as well as brain structures and liver cells together with gastrointestinal organs and thymus glands along with spleen tissue lymph nodes and tonsils and additionally within vascular smooth muscle cells endothelial cells and immune cell populations. Researchers identified NPR-A expression in CD4+T cells following immunomagnetic bead positive selection from mouse spleen cells which suggests ANP functions in immune system regulation. During the investigation of ANP’s role in immune system function researchers found that ANP prevents fetal thymus maturation and differentiation and thymocyte proliferation in mature animals while reducing endothelial cell permeability increases triggered by lipopolysaccharide (LPS) and tumor necrosis factor-α (TNF-α). Apart from cardiovascular and kidney tissues ANP and its receptors appear in other tissues where they exert biological activities affecting endothelial cells and inflammation which suggests that ANP functions beyond blood pressure and water-salt balance regulation to serve as an autocrine or paracrine factor for immune regulation and cytoprotection.
Immune Regulation Function of ANP
ANP/NPR-A is widely involved in immune response. ANP controls immune responses by boosting human neutrophil movement and natural killer (NK) cell killing ability and by suppressing macrophage generation of NO and TNF-α while limiting pro-inflammatory interleukin (IL)-12 actions but enhancing anti-inflammatory interleukin (IL)-10 actions.
ANP and Innate Immunity
ANP assists macrophage phagocytosis against extracellular microorganisms and decreases production of pro-inflammatory markers together with reducing adhesion molecule expression including ICAM-1 and E-selectin.
ANP and Adaptive Immunity
ANP reduces CD4+CD8+T cells while raising levels of CD4-CD8-T cells and induces CD4+T cells to develop into Th2 or Th17 types which then result in tumor cell apoptosis or necrosis.
ANP and DC
As the leading antigen presenting cell (APC) type DC initiates primary immune responses. The generation of intracellular cGMP takes place through ANP’s interaction with NPR-A. Immature DC transform into mature DC with polarized phenotypes for Th1 or Th2 responses when exposed to pathogen-derived compounds along with cytokines and soluble transmitters. Peripheral blood and tonsils contain two primary DC subpopulations which are differentiated based on CD11c expression variations.
ANP and Monocyte-Macrophage
The secretion of cytokines from macrophages is under the control of ANP. Macrophages generate IL-1 which stands out as the most potent pro-inflammatory factor essential for both acute and chronic inflammation. A variety of pathological conditions present with dysregulated expression of IL-1. The body regulates IL-1 production at transcriptional and translational stages because of its crucial function. The NLRP3 inflammasome functions as a critical component of innate immune defense by detecting endogenous and exogenous signals and controlling caspase-1 and IL-1β activity while being involved in several major human diseases including type 2 diabetes and Crohn’s disease along with silicosis, psoriasis, cancer and Alzheimer’s disease. The research revealed ANP/NPR-A/cGMP involvement in pro-IL-1β production through NF-κB and IL-1β release through NF-κB/NLRP3/caspases-1 pathways.
ANP and NK cells
NK cells respond to microorganisms through immune responses and host cell apoptosis while stimulated NK cells release IFN-γ which activates macrophages. The cytotoxic potential of NK cells rises significantly when ANP is applied in vitro yet their population count remains unchanged.