Animal models are indispensable tools for studying the pathogenesis of many diseases, drugs and vaccines. Primates are close relatives of humans and are highly similar to humans in terms of organizational structure, immunity, physiology and metabolism. They are extremely precious and important experimental animals, and their application value far exceeds that of other species of animals. Since the discovery of AIDS, researchers have tried to establish suitable animal models using a variety of primates. Although chimpanzees can be infected with human immunodeficiency virus (HIV), this animal model has limited its practical application due to its limited source, high cost, difficulty in management, and slow progression of disease. Many primates in Africa are naturally infected with the simian immunodeficiency virus (SIV), and SIV can continue to replicate at high levels in the body. However, these natural hosts do not develop AIDS and are therefore not suitable for studying the pathogenesis and evaluating antiviral drugs and vaccines. Infecting Asian monkeys with virulent strains of SIV and constructed simian/human immunodeficiency virus (SHIV) will soon lead Asian monkeys to the AIDS-like illness. The disease development process and multiple detection indicators are very similar to human AIDS. Therefore, this model is widely used in the study of AIDS pathogenesis, anti-HIV/AIDS drugs and vaccines.
The Necessity of Using Animal Models for Anti-HIV Drug Research
HIV remains a major global public health issue, continuing to spread in all countries around the world, killing 40.4 million people so far; new infections are reported in some countries on an increase, compared with a previous decline. As of the end of 2022, there are an estimated 39 million people living with HIV. There is no cure for HIV infection. However, as people gain effective HIV prevention, diagnosis, treatment and care measures, including measures for opportunistic infections, HIV infection has become a manageable chronic health condition and people living with HIV are able to live a long and healthy life.
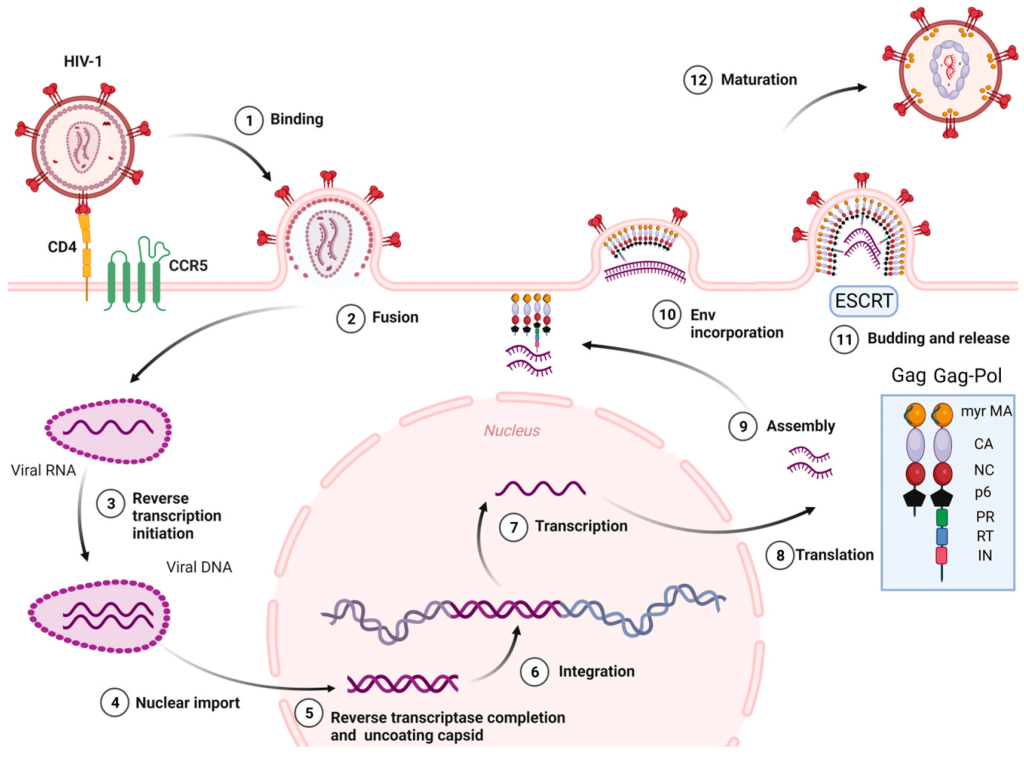
Figure 1. HIV-1 life cycle.
Since no effective anti-HIV vaccine has been developed in the world, antiviral drugs are still the main means of preventing and treating HIV/AIDS. Most anti-HIV drugs are currently developed for the main stages or major enzymes of the virus life cycle (Figure 1), such as virus-cell binding and fusion, viral reverse transcriptase (RT), integrase, protease, etc. As of 2020, the FDA has approved 222 antiretroviral drugs for global HIV/AIDS relief. These drugs can be divided into:
- Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
- Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
- Protease Inhibitors (PIs)
- Fusion Inhibitors
- CCR5 Antagonists
- Integrase Strand Transfer Inhibitor (INSTIs)
- Attachment Inhibitors
- Post-Attachment Inhibitors
- Capsid Inhibitors
- Pharmacokinetic Enhancers
- Combination HIV Medicines
Highly active-retroviral therapy (HAART) has been proven to be an effective anti-HIV/AIDS treatment option in clinical practice. It usually uses a combination of three or more drugs. This treatment strategy can maximize the inhibition of HIV replication. However, HAART treatment has problems such as high cost, inability to clear the virus, large toxic and side effects, easy development of drug resistance, and poor drug compliance. Therefore, there is an urgent need to develop new targets, high-efficiency, low-toxicity, and inexpensive anti-HIV/AIDS drugs.
The process of developing new drugs is a monotonous, time-consuming and expensive process. There are certain limitations in the correlation between anti-HIV pharmacodynamic experiments in vitro and actual conditions in vivo. Most compounds that are effective in vitro testing cannot enter clinical trials due to reasons such as limited sources, large toxic and side effects, poor antiviral effects, and unsatisfactory bioavailability and pharmacokinetics. In clinical trials, setting a placebo control group for AIDS patients is considered unethical, thus seriously hindering the development of new anti-HIV drugs. Therefore, many clinical trials of new anti-HIV drugs are mainly conducted in patients who have failed HAART treatment. These patients usually have low CD4+ T lymphocyte counts, poor drug compliance, and drug resistance, which cannot fully reflect the actual efficacy of the new drug. For the above reasons, using SIV or SHIV-infected primate AIDS models to evaluate anti-HIV drugs and conduct in vivo pharmacodynamic studies has important reference value for suggesting drug development prospects and guiding clinical trials.
Animal Species and Virus Strains Studied for Anti-HIV Drugs
The main animals used in anti-HIV drug research are Asian monkeys, such as rhesus monkeys, cynomolgus monkeys, pig-tailed monkeys, etc. The first SIV was discovered in 1985, and several strains of SIV virus were subsequently isolated from African and Asian monkeys. SIV has certain homology with HIV-1. SIV is divided into five major branches: SIVcpz, SIVsm, SIVmnd, SIVsyk and SIVagm. Inoculation of some SIV virus strains into Asian monkeys such as rhesus monkeys and cynomolgus monkeys will infect them and quickly produce AIDS-like symptoms. Among them, SIVmac and SIVsm are more pathogenic and are the main virus strains used in anti-HIV drug research. These strains are sensitive to many NRTIs, PIs, integrase inhibitors, CCR5-target entry inhibitors, etc., but are not sensitive to NNRTIs.
Using molecular biology technology, some important genes of HIV-1 were recombined with SIV, and a series of SHIV viruses containing HIV-1 and SIV genes were successively constructed and used to infect rhesus monkeys. Initial reports stated that although several strains of SHIV can infect rhesus monkeys, they do not cause disease. Recent studies have shown that several SHIV virus strains can not only infect rhesus monkeys, but also cause them to produce AIDS-like symptoms. SHIV basically maintains the biological characteristics of SIV and carries some HIV-1 antigens. It provides an ideal model for the study of vaccines and biotherapies based on HIV-1 antigen, so it is also widely used in AIDS research. RT-SHIV, which uses the SIV virus genome as the backbone and replaces the SIV reverse transcriptase gene with the HIV-1 reverse transcriptase gene, is sensitive to NNRTIs, making the RT-SHIV/primate model play an important role in evaluating NNRTIs.
Problems in Non-human Primate Models in Anti-HIV Drug Research
The most important problem faced by several widely used AIDS primate models is that they cannot completely simulate clinical HIV infection and that different primates are infected with different immunodeficiency viruses. There are large differences in immune responses, viral loads, clinical symptoms and morbidity. For example, rhesus monkeys infected with SIVmac251 have higher viremia, lower antiviral specific immune response, and faster disease progression. After African green monkeys infected with SIVagm, although they develop high viremia, the animals will still remain healthy. When the same primate is infected with the same immunodeficiency virus, the disease process will also show great individual differences, and the asymptomatic period can last for months to years. Therefore, how to establish an animal model system with stable infection characteristics in order to reduce differences caused by host factors in drug treatment is an urgent problem to be solved. In addition, the difference in drug sensitivity between SIV or SHIV and HIV is also a problem faced by non-human primate models in anti-HIV drug research. Although many SIVs are sensitive to most anti-HIV drugs, in vivo and in vitro studies have shown that some drugs with good anti-HIV effects do not have ideal antiviral effects on SIVs.
The non-human primate (NHP) models have become a powerful tool for HIV/AIDS research. In preclinical pharmacodynamics studies of anti-HIV drugs, this model provides the reference system closest to the actual efficacy of the drug. At the same time, the formulation and testing of optimal treatment plans and treatment strategies is sometimes difficult to carry out clinically, let alone through in vitro experiments. The use of primate models for related research has become the first choice. Creative established rhesus monkey models with SIVmac251, SIVmac239 and SHIV-infected, and carried out evaluation research on the pathogenesis of AIDS, drugs and vaccines, which was recognized by customers. We hope that by carefully designing research plans, our HIV infection rhesus monkey models and non-human primate models can provide more valuable reference and better guide future clinical trials.
Our HIV infection rhesus monkey models:
References
Van Rompay, Koen KA. Tackling HIV and AIDS: contributions by non-human primate models. Lab Animal. 46.6 (2017): 259-270.
Evans, David T., and Guido Silvestri. Nonhuman primate models in AIDS research. Current Opinion in HIV and AIDS. 8.4 (2013): 255-261.
Sever, Belgin, et al. A Review of FDA-Approved Anti-HIV-1 Drugs, Anti-Gag Compounds, and Potential Strategies for HIV-1 Eradication. International Journal of Molecular Sciences. 25.7 (2024): 3659.
