Respiratory syncytial virus was isolated from the cold-like respiratory tract of chimpanzees in 1956. It is called respiratory syncytial virus because it causes the fusion of adjacent cells during cell culture and the formation of syncytial-like structures in the cells. According to different virus species, it is divided into human respiratory syncytial virus (isolated from infant respiratory specimens in 1957), bovine respiratory syncytial virus and murine respiratory syncytial virus.
Epidemiology of Respiratory Syncytial Virus Infection
Respiratory syncytial virus is the most important viral pathogen causing acute lower respiratory tract infections in children under 5 years of age worldwide. Respiratory syncytial virus is the leading cause of severe (and even fatal) lower respiratory tract infections in early postnatal life. It is the main cause of bronchiolitis in infancy and pneumonia in early childhood. Respiratory syncytial virus infection is the leading cause of hospitalization for viral respiratory infections in infants and young children, seriously endangering children’s health, especially premature infants, infants and young children with congenital heart disease or primary immune deficiency.
Pathogenesis of Respiratory Syncytial Virus Infection
The pathogenesis of respiratory syncytial virus infection is complex and involves the combined effects of pathogenic factors, airway epithelial cell-related factors, immune system response, nervous system response, host factors and environmental factors. Respiratory syncytial virus infection is most likely to affect the respiratory system, and its main mechanisms are airway obstruction, bronchial smooth muscle spasm, and subsequent airway hyperresponsiveness.
Respiratory syncytial virus infection can cause airway cilia and airway epithelial cells to shed. The shed airway epithelial cells, neutrophils, cellulose, and lymphocytes accumulate in the airway, causing airway obstruction. At the same time, excessive secretion of mucus and airway obstruction Edema worsens airway obstruction.
The tracheal and bronchial epithelium can be damaged and shed due to inflammatory reactions, resulting in the exposure of sensory nerve endings and the release of active substances, causing bronchial smooth muscle spasm; the exposure of nerve endings causes airway hyperresponsiveness. These mechanisms are mutually responsible and cause a series of symptoms.
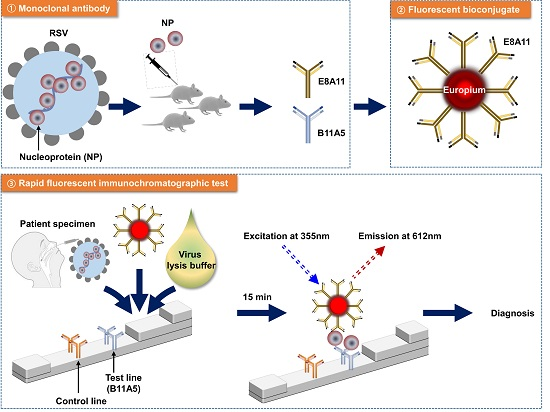
Respiratory Syncytial Virus Detection Method
Currently, technologies such as pathogen isolation and culture, tissue culture, enzyme-linked immunosorbent assay (ELISA), molecular biology, gene chips, and rapid colloidal gold are mainly used to detect Respiratory syncytial virus in clinical practice.
Figure 1. Rapid Fluorescent Immunochromatographic Test to Detect Respiratory Syncytial Virus.
In the early days, culture was a common method for isolating pathogens. This method selects appropriate tissues and cells for inoculation based on the bacterial tropism of the pathogen. Subsequently, immunological and hemoadsorption methods are used to detect protein markers on the surface of infected cells, thereby confirming the detection and identification of pathogen proliferation. Although the virus isolation and culture method can objectively isolate the type of infectious virus, this method is complex to operate, takes a long time to detect, is costly, is prone to false negative results, and the test results are affected by many factors. Therefore, its clinical application rate is not high.
Molecular-level detection has high sensitivity and specificity, but its application in primary medical institutions is limited due to high requirements on laboratory conditions.
Since RSV-specific antibodies are produced slowly in the body and have low titers, the detection sensitivity is not high. Therefore, diagnosing RSV infection by measuring patients’ serum antibodies cannot provide clinicians with a timely basis for diagnosis and treatment. Moreover, the immunology of this method of detecting antibodies the method reflects indirect indicators of infection and cannot replace direct pathogenic testing. Therefore, detecting RSV antigen has become an important way to diagnose the disease in the early stage. However, because the virus titer is low in secretions, it is difficult to diagnose RSV infection by serological methods or antigen examination. The purpose of rapid cell culture centrifugation is to speed up the entry of virus particles into cells, the virus isolation time is much shorter than conventional virus isolation. Compared with direct detection of specimen smears using immunohistochemistry, it increases the proliferation of sensitive cells, which can improve the sensitivity and specificity of detection. This method is especially suitable for viruses with lower titers, but requires the presence of viable virus particles. Early diagnosis of RSV infection should detect both live and dead viruses, that is, cell culture and non-cell culture methods for detecting RSV antigens complement each other. Non-cell culture can detect dead viruses, and cell culture can detect low-titer live viruses.
Related Products
| Cat. No | Product Name | Source/Host | |
| DAG-WT1165 | Recombinant RSV PreF glycoprotein F0 | HEK293 cells | Inquiry |
| DAG-WT1180 | Recombinant RSV PostF glycoprotein F0 | HEK293 cells | Inquiry |
| DMAB-JXL23114 | Anti-RSV-F Reference Antibody (pallvizumab) | Human | Inquiry |
| DMAB-JXL2372 | Anti-RSV Pre-F Monoclonal Antibody, clone D25 | Human | Inquiry |