The endoplasmic reticulum (ER) is an organelle involved in the synthesis, folding, and transport of proteins in eukaryotic cells. It is involved in the regulation of protein homeostasis. Protein homeostasis is a process that monitors the occurrence, folding, assembly, transport, and degradation of proteins. These proteins include those located in the endoplasmic reticulum, the Golgi apparatus, the lysosome, and the plasma membrane, and are involved in the regulation of key cellular processes and protein messengers that mediate intercellular communication. High-quality protein folding is a prerequisite for ensuring cell survival and normal physiological functions. In order to ensure the normal working of the body, strict control mechanisms are needed to ensure the correct folding, modification, and assembly of the protein. Among them, unfolded or misfolded proteins accumulate in the endoplasmic reticulum cavity due to changes in ER homeostasis, called endoplasmic reticulum stress, which activates the unfolded protein response (UPR). UPR activation can promote cell survival or cell death depending on whether ER stress is strong or weak.
Therefore, protein stability in the endoplasmic reticulum protein folding in cells is critical to cell function and survival. There are many processes in the endoplasmic reticulum that help cells maintain a steady state of protein folding. It is well known that the dynamic life cycle of a protein begins with the ribosome. Synthetic peptides usually carry an N-terminal signal sequence that is recognized by a signal recognition particle (SRP). This helps them enter the ER through the transporter complex. After entering the ER, the signal sequence is removed by a signal peptidase, and the nascent polypeptide is modified (eg, by N-glycosylation), assembled and folded to obtain its inherent conformation. At the same time, there are many general and substrate-specific protein disulfide isomerases and peptidyl propylene glycol isomerases in the ER, which can increase the rate of disulfide bond formation and cis-trans isomerization on proline residues and effectiveness. In addition, the endoplasmic reticulum environment of protein folding is very conducive to the folding of proteins and the formation of disulfide bonds due to the oxidative and kilomolar concentration of Ca2 +. The transport of proteins through the early secretion pathway is mainly determined by the composition of the ER companion protein, the protein folding state, and the structure of the N-chain glycan on the polypeptide. It is worth noting that protein folding is the most error-prone step in gene expression.
ER Pressure
Messenger RNA translation is a stage of gene expression that responds rapidly and reversibly to changes in cellular homeostasis and external stimuli through covalent modification of initiation factors. Because protein synthesis and secretion rates vary widely under different cell types and conditions, different levels of nutrition and energy are required to support protein folding needs. In addition, protein folding is also a process that requires energy, requiring coordinated action between organelles and different proteins, as well as resources from multiple metabolic pathways. Therefore, the correct folding efficiency of proteins in the endoplasmic reticulum is highly sensitive to changes in the intracellular environment or extracellular stimuli. Among them, changes in the endoplasmic reticulum homeostasis can lead to the accumulation of misfolded proteins and cause UPR. These include: increased levels of protein synthesis; impaired ubiquitination and proteasome degradation; insufficient autophagy; insufficient energy; excess or restricted nutrition; calcium level imbalance or redox homeostasis; the challenge of inflammation; and hypoxia.
Regulate Signal Path
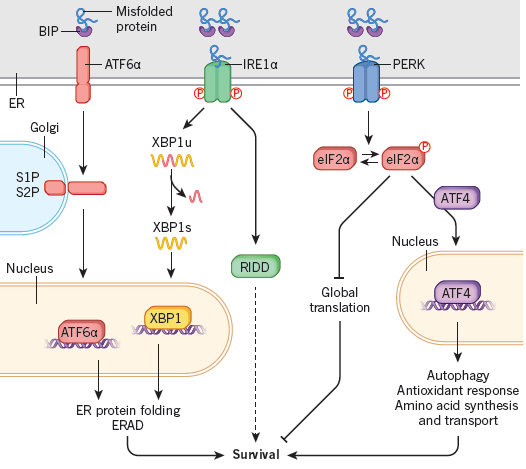
Studies have found that in the endoplasmic reticulum, UPR helps cells adapt to endoplasmic reticulum stress. Existing data indicate that three UPR signaling pathways exist in multicellular animals: PRKR– eIF2α, IRE1α– XBP1, and activated transcription factor (ATF) 6α. Among them, PRKR and IRE1α are type I transmembrane proteins with ER cavity-like domain structure and cytoplasmic serine/threonine kinase domain. ATF6α is a type II transmembrane protein that contains a cytoplasmic loop AMP response element-binding protein (CREB) ATF basic leucine zipper domain. During acute endoplasmic reticulum stress, UPR is activated to restore endoplasmic reticulum protein folding in vivo. This equilibrium process involves transient decay of protein synthesis, increased protein folding and transport in the ER, and related protein degradation and autophagy. To reduce the load on protein folding, activated PERK causes phosphorylation to activate eIF2α, which temporarily suspends the translation of the original messenger RNA. Although total protein synthesis is reduced, eIF2α-P paradoxically up-regulates many mRNA-related genes, such as ATF4, increasing the protein’s ability to transport to the endoplasmic reticulum and other processes in the cell.
ATF6α and IRE1α activate the transcription pathway to increase the ability of cellular proteins to fold, transport and degradation. Under stress conditions, IRE1α is activated. When activated IRE1α enters the nucleus, it not only improves protein folding and transport, but also promotes degradation pathways, and thus can resolve misfolded proteins. Activation of IRE1α also mediates mRNA decay and reduces the protein folding a load of the endoplasmic reticulum. When misfolded proteins accumulate in the ER, ATF6α is transported to the Golgi apparatus and processed to produce a cytoplasmic fragment that increases ER folding capacity mainly by inducing genes encoding protein chaperones. The results show that the three signal branches of UPR are not simultaneously activated by endoplasmic reticulum stress. Under stress conditions, ATF6α and IRE1α activation occurs immediately and then decays over time. PERK is immediately followed by activation and persists exist under chronic ER pressure regulation.
