Ischemic heart disease (characterized by decreased blood supply to the heart muscle) is one of the leading causes of death worldwide. It manifests as a coronary artery occlusion, which in turn leads to myocardial infarction, accompanied by death of myocardial cells. This overloads the surviving heart muscle and eventually leads to heart failure. In addition, other causes can also cause heart failure, including chronic hypertension, which is also characterized by the gradual loss of cardiomyocytes, and experimental inhibition of programmed cell death can improve heart function. Clinically, the effective treatment to solve the fundamental problem of heart loss is heart transplantation. The new discovery that stem cells and progenitor cells have regenerative potential to treat and prevent heart failure has changed experimental research and caused explosive growth in clinical research.
Heart Regeneration
Although heart cells have a slight ability to regenerate. However, it is generally believed that the regenerative capacity of the human heart muscle is seriously insufficient, and it is not enough to make up for the severe loss of myocardium caused by catastrophic myocardial infarction or other heart disease. Studies have found that the heart of some vertebrates (such as zebrafish and salamanders) does undergo a regenerative reaction after injury; under normal conditions, mouse and human cardiomyocytes rarely divide. But after a serious injury, the remaining cardiomyocytes will start DNA synthesis and re-enter the cell cycle. Therefore, the division of existing cardiomyocytes seems to be the most important factor for heart regeneration in mice and humans. The dedifferentiation of cardiomyocytes near the damaged area occurs before their proliferation and is characterized by the loss of expression of myocardial contractile proteins (such as α-myosin heavy chain and troponin T). Studies find zebrafish heart regeneration may be mainly caused by undifferentiated stem cells or progenitor cells from the outer layer of the heart (epithelium). Further research on salamanders and zebrafish will more clearly define whether cardiac regeneration in these organisms requires dedifferentiation, proliferation and subsequent cardiomyocyte differentiation, or whether regeneration is driven by the recruitment of stem cells to the injured site. In contrast, in mammalian hearts, cardiomyocytes rarely divide after a myocardial infarction, although transgene overexpression of specific genes in mice increases the division of cardiomyocytes.
There is strong evidence that endothelial cells are renewed by bone marrow-derived progenitor cells, but the idea that cardiomyocytes are renewed by such cells has been heatedly debated. Less controversial is that adult mammalian heart muscle has a resident cardiac stem cell (CSC) population, which has the potential to differentiate into cardiomyocytes and other cell types (such as endothelial and vascular smooth muscle cells). The study found that CSCs can support the basic turnover of cardiomyocytes, but this turnover occurs at a very low rate without damage. CSCs have high proliferation and differentiation potential in vitro, and it may be a promising therapeutic direction to expand autologous CSCs in vitro or stimulate the regeneration of these cells in vivo.
The recognition that there is indeed a regeneration mechanism in the mammalian myocardium has aroused intense attention. Researchers have discovered that it may hinder the existence of aplastic disorders, including ischemia, inflammation and fibrosis at various stages of myocardial infarction. This unfavorable microenvironment may prevent the activation of resident CSCs, thereby reducing the success rate of exogenous cell therapy. Certain components of the inflammatory response may be essential to promote angiogenesis and progenitor cell recruitment, but excessive inflammation may also prevent the recruitment and survival of progenitor cells. Similarly, after myocardial infarction, a certain degree of fibrosis is required to prevent myocardial rupture, but dense fibrosis presents a strong physical barrier to regenerative cells.
Which Stem Cells Are Used In Heart Therapy?
Perhaps the most confusing aspect of current cardiac regeneration is the different cell types, which are considered to be candidates for cardiac therapy. Multiple cell candidates reflect that human research on cell regeneration is not deep enough, so further research and exploration are needed.
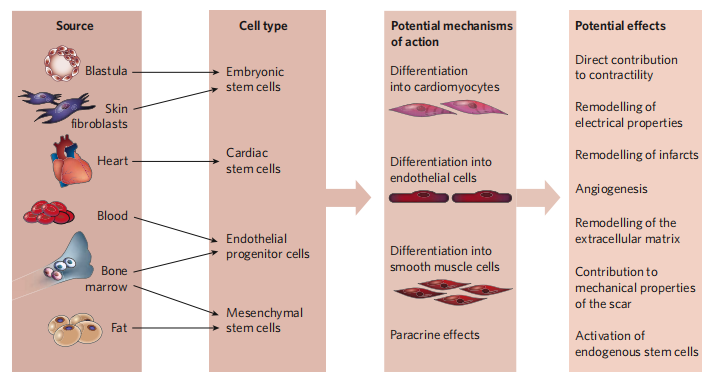
Skeletal Myoblast
One of the earliest cell-based cardiac regeneration strategies was to inject autologous skeletal muscle myoblasts into ischemic myocardium. Myoblasts are resistant to ischemia, and can be differentiated into myotubes (but not into cardiomyocytes) in the laboratory animal experiments and improve ventricular function. The myocardial tube will not integrate with the surviving cardiomyocytes, so it will not beat synchronously with the surrounding myocardium. However, related clinical trials were terminated due to lack of efficacy, so it is unlikely that skeletal myoblasts will actually regenerate the heart muscle. Interestingly, studies found that mouse skeletal muscle contains a large number of non-satellite cells, which can differentiate into spontaneous pulsatile cells with cardiomyocyte characteristics, but no one has found similar cells in human skeletal muscle.
Bone Marrow-Derived Cells
In stem cell cardiac therapy, it was first reported that adult stem cells or progenitor cells transplanted into the infarcted heart of mice that can differentiate into cardiac myocytes are a subset of hematopoietic cells derived from bone marrow. The first evidence that adult bone marrow-derived progenitor cells are involved in the formation of cardiomyocytes in the adult human heart is based on reports of Y chromosome-positive cardiomyocytes in male recipients of transplanted female donor hearts. Animal studies using labeled hematopoietic stem cells for bone marrow transplantation and subsequent myocardial infarction have shown that cardiomyocytes are derived from transplanted cells, but the proportion is extremely low. Moreover, other studies in animals have not demonstrated that hematopoietic progenitor cells can differentiate into cardiomyocytes or improve heart function. Therefore, there is currently no consensus on whether bone marrow-derived progenitor cells differentiate into cardiomyocytes in vivo.
Embryonic stem cell
Embryonic stem (ES) cells are prototype stem cells. They clearly meet all the requirements of stem cells: cloning, self-renewal and multi-potency. ES cells can differentiate into any type of cells present in an adult organism, so it has the potential to completely regenerate the heart muscle. The two obstacles facing the clinical application of ES therapy are immune rejection and the tendency of injecting ES cells to form teratomas. With the increase in knowledge of ES cell differentiation and cardiac embryonic development pathways, ES cell differentiation may become more controllable. Methods to limit teratoma formation include genetic selection of differentiated ES cells, or differentiation of ES cells into cardiomyocytes or endothelial cells in vitro before injection. For example, tumor necrosis factor promotes the differentiation of ES cells into cardiomyocytes. If the differentiated ES cells are delivered to the myocardium in a rich survival mixture, they can survive and improve myocardial function. The inherent difficulty in controlling the growth and differentiation of ES cells and other pluripotent stem cells is that the timing of activating specific signaling pathways may be crucial. For example, recent studies on mouse and zebrafish embryos have shown that the role of the Wnt-β-catenin pathway in heart development depends on the stage of development.
Endogenous cardiac stem cells
Because allogeneic cells face immunological challenges that may require immune rejection, the isolation of endogenous adult mammalian CSCs based on cell surface markers has attracted great interest. However, no clear CSC mark has been determined so far. Mammalian heart muscle includes a small percentage of stem cells expressing cell surface markers Kit or Scal. In addition, some side population cells also express Kit and / or Sca1, and like Kit + CSC and Sca1 + CSC, side population cells can produce cardiomyocytes in vitro and in vivo. In addition to Kit + CSC, Sca1 + CSC and side population cells, the fourth type of CSC also expresses the transcription factor Isl1. The tracer experiments showed that during embryonic heart development, cells expressing Isl1 can differentiate into endothelial cells, endocardial cells, smooth muscle cells, conduction system cells, right ventricular myogenic cells and atrial myogenic cells. There are also cells that express Isl1 in the heart of adult mammals, but they are limited to the right atrium, are found in fewer numbers than the embryonic heart, and have unknown physiological effects. Recently, epicardial-derived progenitor cells with angiogenic potential have been described.
Stem cell therapy for heart disease faces some challenges. The most important question to be answered in preclinical research is which stem or progenitor cells are the best choice for treatment. So far, under certain circumstances (acute myocardial infarction), bone marrow-derived progenitor cell therapy has proven to be safe and beneficial, but the regeneration potential of this cell is still controversial. CSC may have the potential to target patients, but isolation and cultivation procedures are still in the early stages of development. ES cells have the greatest differentiation potential, but face moral barriers and the greatest risk of teratoma formation. Whether ES cell derivatives will be rejected by the host’s immune response is still under debate. However, in principle, rejection can be avoided by using cells from a pool of only 150 donors with different HLA types. If new technologies for reprogramming human and mouse fibroblasts into ES-like cells can be used, the use of patient-reprogrammed cells can reduce or even eliminate immune rejection. When designing a more rational cell-based treatment for heart disease, a key issue is to understand the mechanism by which each stem or progenitor cell type can affect myocardial function. Similarly, different cardiology, such as acute myocardial infarction and chronic ischemic cardiomyopathy, may require different types of stem or progenitor cells.
