During mitosis, transcription is stopped and many chromatin characteristics are lost, which poses a challenge to the continuity of cell identity, especially in fast-circulating stem cells, which require a constant balance between self-renewal and differentiation. If a cell doesn’t remember what it should be, it can turn into something else, even a malignant cell. So understanding how to control this process is critical to understanding related disease. How does the stem cell retain its cellular identity?
Previous studies have shown that cell identity is determined by characteristic gene expression programs and chromatin distribution, which are constantly monitored by key transcription factors (TF), which are called master regulators. This tightly controlled system is temporarily unstable during mitosis, when dramatic molecular changes occur, such as global transcriptional shutdown, large changes in histone modifications, and the dissociation of most transcription factors and cofactors from mitotic chromatin. . How to faithfully restore cell type-specific procedures in daughter cells is a fundamental but unanswered question in biology. Studies in different somatic cell types have shown that the heritability of defined gene expression programs may depend on epigenetic markers (histone and DNA modifications) and/or mitotic persistence of TF on chromatin, a phenomenon known as mitosis Bookmark. There are some results show that partial retention of specific active or inhibitory histone markers so that the corresponding histone readers and authors can be quickly recruited after G1 entry, thereby ensuring self-renewal of gene expression status. There are also many studies showing that selected TFs are still associated with mitotic chromatin, thereby facilitating rapid activation of key genes for their respective cell identities. Overall, these suggest that both epigenetic and TF bookmarking mechanisms contribute to the faithful spread of cell identity after cell division.
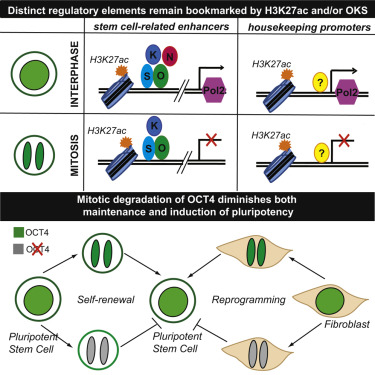
Among the many cell types that study cell identity retention, pluripotent stem cells are the most representative. Pluripotent stem cells (PSCs) not only have unlimited self-renewal capacity, but also retain the potential to differentiate into all somatic cell types based on developmental cues. In addition, PSC is characterized by a very fast cell cycle (10-12 hours), a lack of G0 phase, and an abnormally short G1 phase. This requires the existence of a very efficient mechanism for preserving or resetting PSC-specific transcription patterns. It is worth noting that there is a well-defined TF network that controls the maintenance and acquisition of stem cell identity. According to a new study published by Weill Cornell medical scientists, it was found that the stem cell-associated gene enhancer is labeled with H3K27ac and the main regulators OCT4, SOX2 and KLF4, while The promoter of the cell cycle regulatory gene is also labeled by H3K27ac during mitosis. In addition, the transient degradation of OCT4 at the end of mitosis also impairs the ability of pluripotent stem cells to maintain and induce pluripotency, indicating that pluripotent stem cell identity maintenance is partially dependent on its bookmarking activity. These results indicate that histone modifications and transcription factors are involved in the regulation of faithful and efficient reproduction following stem cell division.
This finding is crucial because pluripotent stem cells have attractive potential for the prevention and treatment of many diseases. While it is fundamental to understanding disease, as many diseases, including cancer and some neurological diseases, are the result of loss of cellular identity. If we can learn more about how cells maintain their identity in the process, we will better understand the formation of tumors and even can push stem cells to a therapeutically relevant identity to a particular disease.
