Staphylococcus aureus (S. aureus) is an important class of food-borne pathogens that cause food contamination and food poisoning, and is also a symbiont of human skin and mucous membranes. Studies have found that about 20%~30 % of healthy individuals carry Staphylococcus aureus. Animal foods are susceptible to Staphylococcus aureus infection, such as milk, butter, cured ham, etc. Food poisoning incidents caused by Staphylococcus aureus have been frequently reported, and more than 95% of them are caused by enterotoxin. According to reports, the United States and Canada accounted for 33% and 45% of food poisoning incidents caused by staphylococcal enterotoxins (SEs), respectively. Epidemiological studies have shown that Staphylococcus aureus enterotoxin A can be produced under more suitable environmental conditions (temperature 7~47, pH 4℃~10) and growth phase (logarithmic growth phase or transition to stable phase) Staphylococcal enterotoxin A(SEA), Staphylococcal enterotoxin B (SEB), Staphylococcal enterotoxin C1 (SEC1), Staphylococcal enterotoxin D (SED) and other about 23 serotypes, they are similar in structure and function with molecular weight of 27.5~30 kDa. Among the above-mentioned SEs, SEB is one of the most common biotoxins reported so far, and it is also a typical biotoxin that causes food poisoning. In order to detect SEB, researchers have developed different detection methods, among which the main detection methods are biological methods, immunological methods, gene probe methods, instrumental analysis methods and biosensing methods.
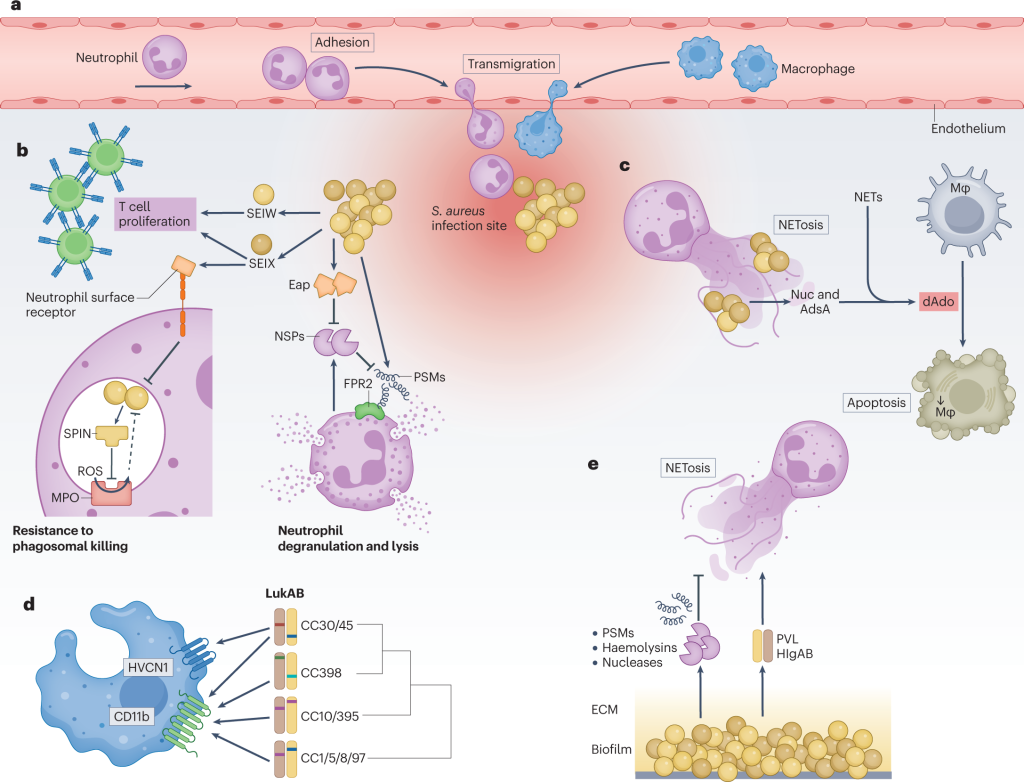
Figure 1. New insights into Staphylococcus aureus immune evasion.
Structural Properties and Hazards of SEB
SEB is mostly found in spoiled milk and meat products in food. According to relevant research reports, the content of SEB in the culture supernatant of Staphylococcus aureus (strain S-6) is as high as 0.2 mg/mL, and the semi-lethal dose LD50 of mice is 0.02 µg/kg. The molecular weight of SEB is 28.4 KDa, which contains 239 amino acid fragments. The structure of SEB is mainly composed of 2 regions, of which 129 amino acid residues in region 1 contain 2 β-sheets and 3 α-helices (α1, α2 and α3). The 2 β-sheets form a cylindrical structure, the inner wall is lined with hydrophilic residues, and they form “crosses” with each other. Region 2 is divided into 2 different parts, one part contains 2 α-helices (α4 and α5) and 2 very short β-strands, and the other part has α-helices located between the curved sheet and β cylinder. One side of α4 and α5 is exposed to the solvent, and the two domains are connected by a ring structure. Compared with other enterotoxins, SEB has stronger heat resistance, its structure and function do not change significantly after being heated at 100 ℃ for 30 min, and it can resist the hydrolysis of intestinal proteases. Therefore, enterotoxin-contaminated food can still cause potential toxic effects even after being cooked and digested by the human body.
As a superantigen substance, enterotoxin B simultaneously forms a bridge by cross-linking with major histocompatibility complex (MHC) class II molecules on antigen-presenting cells (APCs), Stimulates T cells in a manner that does not involve antigen recognition by generating receptor complexes with MHC II sites on APCs and T cells. It can effectively bypass the treatment of conventional APCs, induce T cells to produce cytokines, and cause the activation and proliferation of T cell subsets. Related studies have further confirmed that SEB increases the expression of components of the arachidonic acid pathway, leading to inflammation, edema and shock. Current studies have found that ingesting a small amount of SEB in the human body can cause immune disorders, organ damage and other symptoms, and the clinical manifestations are fever, respiratory diseases (cough, dyspnea and chest pain) and digestive tract diseases. Therefore, it is of great significance to study the highly sensitive and rapid detection method of SEB for the clinical diagnosis, prevention and control of food poisoning.
Method for the Detection of Staphylococcus aureus Enterotoxin B
Biological Detection Method
The detection method of Staphylococcus aureus enterotoxin B was initially based on related detection methods such as zoological experiments. Through intraperitoneal injection and feeding of samples added with enterotoxin B to the tested animals, the abnormal physiological reactions and activities of the animals were observed and recorded. The existence and toxicity of enterotoxin B are qualitatively analyzed. This type of experiment is intuitive in the interpretation of the results, but the disadvantages such as poor specificity limit the application of this method.
Immunoassay
Immunological detection is mainly based on the principle of specific combination of antigen and antibody, including immunoagglutination test, agarose diffusion method and enzyme-linked immunosorbent assay. However, the detection sensitivity of these methods is only 0.1 ng/mL, and the sample preparation and detection take a long time. In addition, this method needs to ensure the homogeneity of monoclonal antibody structure and avoid cross-reaction between each other.
- Immunoagglutination Test
The immunoagglutination test is the agglutination produced by the combination of antigen and antibody in the presence of electrolytes. These include the reverse indirect hemagglutination test and the reverse passive latex agglutination test. The former uses the antiserum of enterotoxin B to adsorb on the surface of red blood cells of animals. When the test specimen contains SEB, hemagglutination occurs. This method can interpret the results in about 2 hours, and the detection sensitivity is greatly improved compared with the previous method; the latter adsorbs the specific anti-SEB antibody on the latex particles, and when the electrolyte and SEB exist at the same time, the latex particles agglutinate phenomenon, the detection time is about 18 h.
2. Agarose Diffusion Method
Immuno-agar diffusion method refers to the diffusion of soluble antigens and antibodies in semi-solid agar. It is not suitable for the detection of low concentration SEB (below ng level). If the antigen and antibody react according to a certain concentration relationship, a white precipitate line appears on the surface of the plate, which is a positive result, and the standard curve is made by the logarithm of the distance of the SEB diffusion agarose layer and the antigen concentration, which is used for the indirect quantification of SEB . The method is simple to operate and does not require any equipment, but the disadvantages of long detection time and low sensitivity limit the application of this method.
3. Immunolabeling Technique
Immunolabeling techniques can be divided into detection techniques such as enzyme-linked immunosorbent immunoassay, radioimmunoassay, and immunofluorescence. It is based on the traditional antigen-antibody reaction, by modifying fluorescent groups on antibodies such as hydroxyfluorescein (financial analyst meeting, FAM), Fluoresceine isothiocyanate (FITC), water-soluble indocyanine-type fluorescein (cyanine-5, Cy5), etc., radioactive isotopes, horseradish peroxidase (horseradish peroxidase, HRP) or alkaline phosphatase (alkaline phosphatase, AP) and other trace substances, using relevant equipment to output signal intensity to achieve the purpose of detection.
Enzyme-linked Immunoassay Technology
Enzyme linked immunosorbent assay (ELISA) is based on the antigen-antibody reaction of a solid-phase carrier (such as polystyrene, etc.), and the color development of the substrate is catalyzed by HRP or AP, thereby amplifying the detection signal. Double-antibody sandwich and indirect competition methods are usually used to detect SEB, and the former can obtain lower detection sensitivity and wider linear range. At present, enzyme-linked immunoassay technology has been widely used in actual detection, and a targeted detection kit has been developed.
Radioimmunoassay Technology
Radioimmunoassay is based on the competitive reaction of labeled antigen (labeled with radioactive isotope) and non-labeled antigen to antibody, and is used to detect the antigen concentration of the sample to be tested.
Immunofluorescence Detection Technique
Immunofluorescence detection technology uses fluorescent substances (FITC, Cy5, quantum dots, upconversion particles, etc.) as signal molecules labeled on antibodies, and the concentration of SEB is indirectly measured by the fluorescence intensity of the labeled antibody bound to SEB, usually using quantitative determination by photometer.
Colloidal Gold Test Strip Detection Technology
Colloidal gold-labeled immune test strip mainly utilizes the excellent physical and chemical properties of colloidal gold. Since the surface of colloidal gold has a negative charge, under alkaline conditions, the antibody can be immobilized on the surface by physical adsorption without affecting the biological activity of the antibody itself. This method can quickly interpret the results under naked eye conditions, and is generally used for preliminary screening on the spot.
Gene Probe Method
Enterotoxin B is transcribed and translated from the toxin expression gene sequence seb of Staphylococcus aureus. By detecting the enterotoxin B gene in Staphylococcus aureus in food, it can be used for rapid and high-throughput detection of positive strains of Staphylococcus aureus. Specifically, the method mainly uses nucleic acid amplification techniques, including polymerase chain amplification and nucleic acid isothermal amplification techniques.
Instrumental Analysis Method
With the continuous improvement of the current detection instruments and equipment, many scholars are no longer limited to the traditional immunology and other related methods, but instead use modern large-scale precision instruments for qualitative and quantitative detection of enterotoxin B. Some instrumental analysis methods can directly reveal the molecular structure of toxins.
Biosensing Detection Method
Biosensor (biosensor) connects biochemical reactions with transducers through physical and chemical instruments, and expresses them in the form of electricity. It mainly recognizes biological components of specific substances (such as enzymes, antibodies, nucleic acids, lectins, Cells or tissues) and biological reaction signal amplification are organically combined, which has the advantages of less sample usage and fast analysis speed, and can be used for simultaneous detection of multiple substances. At present, the biosensors used for detection mainly include electrochemical sensors, surface plasmon resonance and piezoelectric crystal sensors.