It has been clinically found that polyamine biosynthesis is often dysregulated in human cancers, especially in the development of early colorectal cancer. Recent research on polyamine metabolism has made new progress. Researchers at the University of Kentucky have discovered that spermine synthase (SMS) can promote the growth of colorectal cancer.
Studies have shown that SMS is an enzyme that produces spermine from spermidine. It is very important for cell growth. Because the excessive accumulation of spermidine can have a harmful effect on cell viability, the body has a precise mechanism to regulate the level of spermidine in the cell. However, the mechanism of tumor cells maintain a relatively high level of spermidine and below the toxicity threshold to promote tumor growth remains unclear.
Recently, researchers at the University of Kentucky found that SMS is overexpressed in colorectal cancer (CRC) and plays an important role in balancing the level of spermidine in cells. In this study, the researchers discovered that SMS and MYC together inhibit the important function of the apoptotic protein Bim by a unique regulatory pathway, and found that the down-regulation of Bim is a key survival signal for promoting CRC tumor growth.
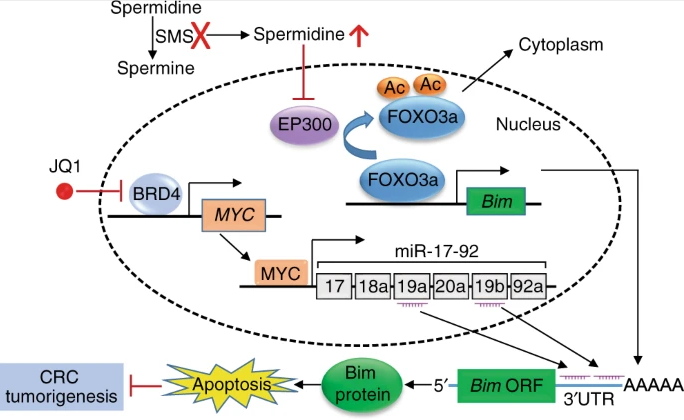
Studies have shown that SMS overexpression plays an important role in balancing cell spermidine levels, and low spermidine levels are a necessary condition for the occurrence of CRC. In normal body cells, polyamine homeostasis is necessary for cell growth and tissue regeneration. With age, in model organisms and in humans, due to the decline in polyamine biosynthesis, the concentration of spermidine in tissues also decreases. In contrast, oncogenic signals induce the up-regulation of the biosynthetic activity of polyamine generating enzymes (such as ODC, SRM, SAMDC, and SMS), which in turn leads to an increase in the level of polyamines, which are thought to play an important role in tumor development. However, excessive polyamine accumulation itself can have a serious and harmful effect on cell activity. Similar to these findings, SMS is overexpressed in CRC during the entire tumorigenesis process, which can destroy the excessive accumulation of spermidine, and then make the spermidine in CRC cells at a tolerable level. Under normal physiological conditions, normal cells with properly regulated polyamine homeostasis can tolerate a certain level of spermidine, which is beneficial to their proliferation. In CRC cells, since the up-regulation of polyamine biosynthesis enzymes is much higher than in normal cells, spermidine is converted into spermine to prevent the level of spermidine from exceeding the toxicity threshold. Therefore, SMS gene knockout completely inhibits the function of SMS and induces excessive accumulation of spermidine, which leads to the inhibition of the growth of CRC.
Further studies have found that the targeted destruction of SMS in CRC cells leads to the accumulation of spermidine, which transfers to the nucleus and transcriptionally induces the expression of the pro-apoptotic protein Bim. However, myc-driven expression of miR-19a and miR-19b inhibited the production of Bim, thereby attenuating this induction. In CRC cells lacking SMS, drugs or genes inhibiting MYC activity can significantly induce Bim expression and apoptosis, leading to tumor regression, but these effects will be significantly reduced after Bim is silenced. These findings reveal the key survival signals in CRC that inhibit the expression of Bim through the aggregation of different signals and myc-mediated signaling pathways.
Therefore, inhibiting SMS and MYC signaling is a promising treatment strategy for CRC. The Cancer Genome Atlas data shows that MYC-dependent transcription is activated in almost all CRCs. Therefore, MYC is an attractive therapeutic target for CRC. Although there is no clear ligand-binding domain small molecule drug for MYC, emerging evidence suggests that small molecule compounds (such as JQ1) that target the epigenetic readers of the bromodomain and terminal extradomain (BET) family act as inhibitors of MYC activity. However, the efficacy of BET inhibitors in CRC is generally moderate, indicating that CRC tumors are inherently resistant to BET inhibition. However, the combined use of SMS inhibitors and BET inhibitors may have broad prospects in the treatment of CRC that is abnormally affected by SMS and MYC-mediated signaling pathways.
