RNA modification is a dynamic, reversible and widespread epigenetic regulatory mechanism. Currently, there are more than 170 known RNA chemical modifications. RNA modification can occur on both coding RNA and non-coding RNA, and constitutes an important part of the “epitranscriptome” in cells. Among them, methylation is one of the most important RNA modifications and is also a hot topic of current research. Since RNA methylation modification participates in the regulation of the shearing, transport, stability, structure and translation efficiency of many RNAs in cells, it widely mediates gene expression regulation and multiple physiological and pathological processes. RNA methylation modification can also directly participate in the regulation of DNA damage repair process, thereby regulating the occurrence and development of tumors from another aspect, and plays an important role in chemotherapy resistance.
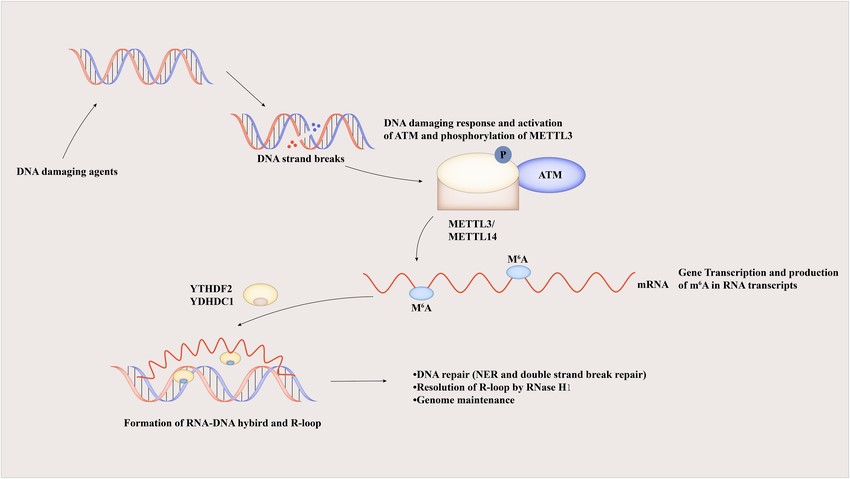
Figure 1. M6A regulates DNA repair through the R-loop to maintain genomic stability.
Definition and Types of RNA Methylation Modification
In nature, RNA modification is widely present in a variety of nucleotides, such as A, U, C, G, and I, of which methylation modification accounts for about 2/3 of the total modification. In eukaryotes, there are many RNA methylation modifications, such as N6-methyladenosine (m6A), 5-methylcytosine (m5C), N7-methylguanosine (m7G), N6,2-O-dimethyladenine (m6Am) and N1-methyladenosine (m1A).
m6A
m6A refers to the methylation that occurs on the N atom at position 6 of base A and is involved in many important links in normal and abnormal biological processes such as RNA shearing, translation and degradation. It has been shown that m6A modifications are mainly distributed in the coding region of mRNA, near the splice site, the stop codon region and the 3′ non-coding region (3’UTR). At the same time, m6A is also present in the exon region, which has the conservation of the RRACH sequence (R is usually G and A, H is usually A, C and U). The most classical sequence is GGACU.
Three types of proteins are involved in the m6A modification of RNA: methyltransferases (writers), demethylases (erasers) and methylation readers. In essence, it is a dynamic and reversible process in which m6A is formed by methyltransferases, removed by demethylases, and the modified sites can be recognised by methylation readers. Previous studies have shown that m6A modification affects the stability of the mRNA itself, the efficiency of protein translation, chromatin remodelling and histone modification. Studies have shown that during RNA transcription, m6A modification can directly demethylate adjacent DNA, thereby increasing chromatin accessibility and the expression of the genes in which it is located. In addition to its function in regulating gene expression, abnormal m6A methylation levels can also cause dysregulation of downstream gene expression, leading to a variety of diseases including tumours, cardiovascular dysfunction and Alzheimer’s disease.
m5C
m5C is also a dynamic and reversible modification that is widely present in rRNA, mRNA, tRNA and many non-coding RNAs. The m5C modification in mRNA is mainly enriched in the untranslated region (3’UTR and 5’UTR), GC-rich region and near the AGO protein binding site with an AUCGANGO motif. RNA m5C methylation modification can mediate a variety of biological functions, including RNA output, ribosome assembly and translation. Similar to m6A, m5C also involves writers, erasers and readers.
m7G
m7G was first discovered in eukaryotic mRNA, tRNA and rRNA. Its most typical enzyme is METTL1, and other related enzymes are less studied. The m7G modification in mRNA is enriched at the 5’UTR and can be dynamically regulated with changes in stress. Its function is to promote translation. In rRNA, m7G modification is mediated by WBSCR22, but its role needs to be further investigated.
DNA Damage Repair and Tumorigenesis
Causes and Repair Methods of DNA Damage
DNA is constantly attacked by exogenous and exogenous factors in cells and is damaged, resulting in genomic instability. This genomic instability is one of the important reasons for promoting the occurrence and development of tumors. Different damaging factors will cause different DNA damage. DNA damage mainly includes DNA single-strand and double-strand breaks (DSB), base mismatches, and interstrand crosslinks, among which DSB has the greatest toxic effect on cells. In order to maintain the integrity of the genome structure, cells will respond to different types of DNA damage through a variety of repair methods such as direct repair, base excision repair, nucleic acid excision repair, mismatch repair, homologous recombination repair (HRR), and non-homologous end joining (NHEJ). HRR, as the most important and precise repair method for DNA double-strand damage, occupies an important position.
Abnormal DNA Damage Repair and Tumor Treatment
Abnormal DNA damage repair in cells can lead to DNA mutations, endanger genome stability, and thus mediate the transformation of normal cells to malignancy. Studies have shown that the DNA damage repair pathway is one of the tumorigenesis pathways, and defects in DNA damage repair can promote tumorigenesis. Compared with normal tissues, cell proliferation in tumor tissues is abnormal and the degree of DNA damage increases. In this case, the apoptosis gene P53 is inactivated and the driver gene is activated, resulting in increased DNA replication pressure and an increase in the incidence of DNA damage, especially DSB. After DNA damage, on the one hand, ataxia telangiectasia-mutated (ATM) protein kinase, ATM and Rad3-related (ATR) protein kinase, etc. quickly sense the damage and start the DNA damage repair mechanism, activate the tumor suppressor gene P53, and induce cell apoptosis; on the other hand, the accumulation of DNA damage causes genomic instability, increases the incidence of tumors, and ultimately causes normal tissues to develop into malignant tumors.
Regulation of DNA Damage by RNA Methylation Modification and Chemoresistance
DSB is the most cytotoxic DNA damage. If not repaired in time, it will damage genome stability and chromosome integrity. In mammals, there are two main pathways for DSB repair: homologous recombination (HR) and NHEJ. Related studies have shown that RNA plays an important role in DNA damage response (DDR), especially dilncRNA and DDRNA have been reported to exist at DSB sites, thereby promoting DNA double-strand breaks repair (DSBR). At the same time, more evidence shows that dilncRNA can form DNA-RNA hybrid double strands at DSB sites, thereby promoting DNA repair proteins such as breast cancer susceptibility protein 1 (BRCA1), BRCA2, DNA repair protein RAD51 and meiotic recombination protein 11 (MRE11) to approach the proximal DSB site, thereby improving the efficiency of DSB repair. In addition, DSBs in the transcriptionally active regions of the genome can also induce the formation of DNA-RNA hybrid double strands, and the induction of reactive oxygen species can also play the same role.
