Over the past few decades, anti-tumor treatment has gradually evolved from traditional surgical resection, radiotherapy and chemotherapy to more precise molecularly targeted therapy and immunotherapy. These advances have improved the treatment of certain tumors to a certain extent. However, effective treatments are still limited for many tumors that are difficult to treat and prone to recurrence. In particular, for solid tumors, the complexity of the tumor microenvironment and the heterogeneity of tumor cells have a significant impact on therapeutic response, often leading to poor therapeutic outcomes and the development of treatment resistance. Faced with many challenges, scientists have begun to explore novel treatment options. Cell therapy, particularly adoptive cell therapy (ACT), has shown great potential by enhancing or modifying a patient’s own or donor cells to attack tumor cells. Among them, chimeric antigen receptor (CAR)-modified T-cell therapy (CAR-T), as a representative of ACT, has made remarkable achievements in anti-haematological tumor treatment, marking the advancement of cell therapy in the field of tumor treatment. Despite challenges such as high cost, technical complexity and potential side effects, cell therapy has become the focus of anti-tumor treatment research, indicating that treatments will evolve in a more personalised and effective direction. This review comprehensively introduces the history of CAR technology and systematically describes the research progress, challenges and future development directions of various CAR-engineered cell therapies in clinical trials.
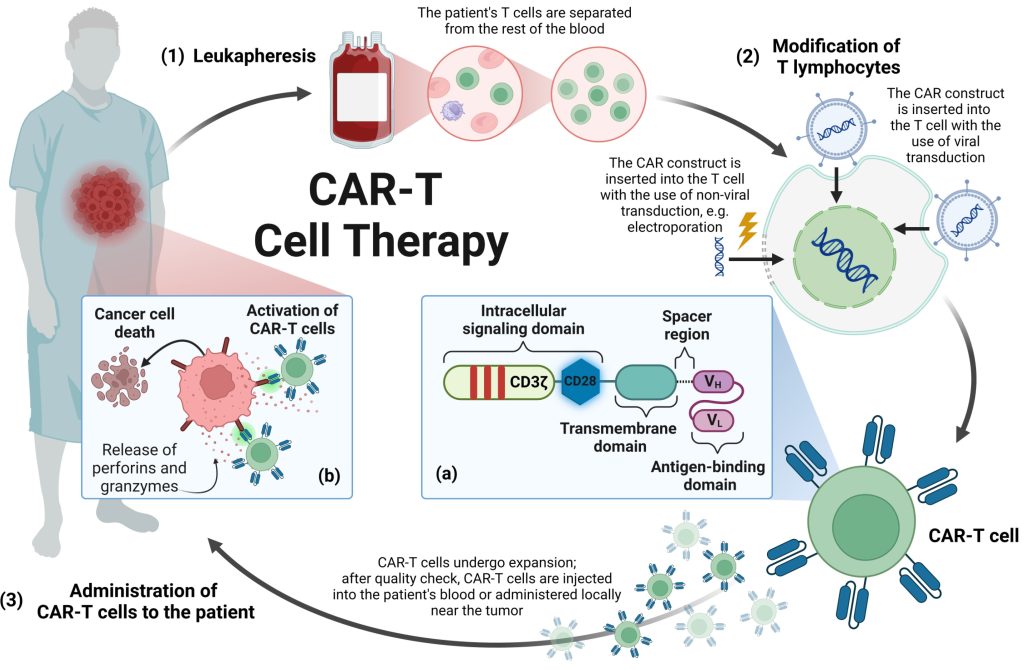
Figure 1. Manufacturing process of CAR-T cell therapy.
CAR Structure
CAR is a specially designed synthetic protein used to enhance the ability of cells to specifically recognize and eliminate tumor cells. It consists of three main parts: the extracellular domain, the transmembrane domain, and the intracellular domain. The extracellular domain mainly includes a single-chain variable fragment (scFv) and a connecting hinge region. scFv is an engineered protein structure that helps CAR-T cells recognize and bind to specific markers on the surface of tumor cells, also known as tumor-associated antigens (TAA). This recognition mechanism is independent of the major histocompatibility complex (MHC) system, thereby helping immune cells bypass the MHC-mediated immune escape strategies that tumor cells may adopt. The connecting hinge region is a key connection site, usually derived from molecules such as IgG or CD8α/CD28. The length and structure of this region can be adjusted to regulate the CAR signal strength and immune cell function. The transmembrane domain has an important influence on the expression and stability of CAR on the cell surface, and is also a key factor in signal transmission efficiency and CAR-T cell function. Commonly used transmembrane domains are derived from proteins such as CD4, CD8α, and CD28. The intracellular domain contains a co-stimulatory domain and an activation domain. Among them, the key role of the co-stimulatory domain is to enhance the activity and persistence of CAR-modified immune cells, such as CD28 promoting T cell proliferation and initial killing function, and 4-1BB prolonging the survival time of modified cells and enhancing their memory function. The activation domain usually uses the CD3ζ chain, which contains a key signaling element, the immunoreceptor tyrosine activation motif (ITAM). When CAR binds to the target antigen, these elements can transmit activation signals to CAR-T cells, thereby prompting them to perform immune functions, such as secreting cytokines and directly killing tumor cells.
Application of T Cells in CAR
T cells are an important component of circulating lymphocytes, play a central role in the human immune system and have a variety of surface molecular characteristics. These characteristics allow T cells to be classified into several subtypes according to their function, including, but not limited to, initial T cells, cytotoxic T cells, helper T cells, regulatory T cells and memory T cells. According to the different compositions of T cell surface receptors (TCRs), T cells can be further classified into αβ T cells (expressing TCRs composed of α chains and β chains) and γδ T cells (expressing TCRs composed of γ chains and δ chains). Among these, αβ T-cells are the most widely studied T-cell subtypes, especially the CD8+ cytotoxic T-cell subtype within them, which can directly kill tumor cells and is also the most commonly used subtype of CAR-T cells in anti-tumor therapy. CAR-T therapy targeting tumor cells is the earliest and most widely used form of CAR technology. Rosenberg originally proposed the concept of using a patient’s own immune cells to attack tumor cells and demonstrated this idea in experiments using tumor-infiltrating lymphocytes (TILs) from melanoma patients.
To overcome the problem of low immunogenicity of certain tumors, Israeli scientist Professor Zelig Eshhar developed CAR technology and created the first generation of CAR-T cells that can bypass MHC restrictions. He fused scFv corresponding to specific TAA with FcεRI receptor (γ chain) or CD3 complex (ζ chain) to construct CARs, and successfully expressed these CARs on the surface of T cells using genetic engineering technology, so that T cells can directly and specifically recognise and combine with TAA to kill tumor cells. However, these cells are unable to maintain long-term viability. To overcome this limitation, Carl H. June’s team developed second-generation CAR-T cells, which significantly increased their efficacy by introducing a costimulatory domain (such as CD137). The first patient with acute lymphoblastic leukaemia to be treated with second-generation CAR-T cells has survived more than 10 years since treatment in 2012, becoming a miracle in the history of medicine. The third generation of CAR-T cells attempts to increase activity by adding a co-stimulatory domain to the second generation, but studies have shown that their anti-tumor efficacy is not significantly better than the second generation. The fourth generation of CAR-T cells introduces specific cytokines or suicide genes to improve therapeutic efficacy while controlling potential toxicity. The fifth generation aims to achieve large-scale production of universal CAR-T cells to improve the availability and cost-effectiveness of treatment, but it must overcome higher technical barriers and meet more stringent safety requirements.
Although CAR technology continues to innovate and evolve, second-generation CAR technology remains the preferred technology for cell transformation. To date, ten CAR-T cell therapy products have been approved by the FDA for worldwide marketing, but the CAR-T cell therapies currently on the market are all for blood cancers. In the treatment of solid tumours, the clinical effect of CAR-T therapy has not yet met expectations, indicating that CAR technology and CAR-T therapy still face major development challenges.