Antibodies are biological protein molecules that are produced by immune B cells after being stimulated by antigens that can specifically bind to the antigen, and then neutralize the antigen. Because antibodies can specifically bind antigens with high affinity, antibodies have always been one of the core molecules in the field of biomedicine and are widely used in academic research, disease diagnosis, and medical drugs.
The traditional antibody molecule (IgG) is a protein composed of two identical heavy chains and two identical light chains with a fairly conservative structure. The light chain of an antibody molecule contains 1 VL region and 1 CL region, while the heavy chain has 1 VH region and 3 CH regions (CH1, CH2, and CH3).
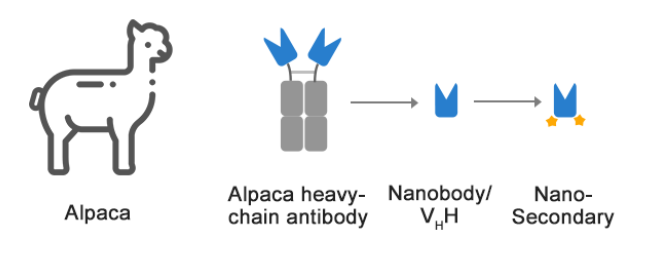
Single domain antibody (sdAb), also known as nanobody (nanobody). It is a special antibody composed of only two heavy chains that naturally occurs in camelids and cartilaginous fishes. It contains only one heavy chain variable region (VHH) and two conventional CH2 and CH3 regions. Single-domain antibodies bind antigens through a variable region (VHH) on the heavy chain, which can exist independently and stably in vitro, and are called camelid single-domain antibodies (SdAb) or nanobody (nanobody). Nanobody crystals are 2.5nm wide and 4nm long. The molecular weight is only 1/10 (about 15kD) of traditional intact antibodies, but they still have complete antigen recognition capabilities. The complete antibody sequence is generally obtained through phage screening. The individually cloned and expressed VHH region has the structural stability and the same antigen binding activity as the original heavy chain antibody. VHH is the smallest unit known to bind the target antigen.
Advantages of Nano Secondary Antibodies
Nano secondary antibodies developed based on single domain antibodies provide researchers with many advantages. Unlike traditional secondary antibodies, small nanobodies will not oligomerize with the primary antibody when incubated together. On the bench, this can shorten the staining step because the primary antibody can be “labeled” by incubating the primary antibody with the labeled Nanobody. After the pre-incubation step, the first antibody-nanobody mixture can be added to your sample, thus eliminating the incubation of the second antibody. In addition, nano secondary antibodies are about 10 times smaller than conventional secondary antibodies. The small size can achieve better tissue penetration, antigen entry, and reduce the distance between the epitope and the label. Therefore, they are ideal probes for super-resolution microscopy. Similarly, Creative Diagnostics Nano secondary antibodies select only Nanobodies with the required specificity during the development process. Eliminates cross-reactivity with other commonly used species of IgG and other IgG subclasses. This combination of high specificity and extremely low background makes the immunoassay more applicable and reduces the challenge of immunoassay (such as multiple western blotting or multiple super-resolution microscopy detection, even in pre-adsorbed mouse serum Later, it is also common to see cross-reactions between mouse IgG subclasses.). Our nanosecondary has ultra-high specificity and therefore does not require any type of pre-adsorption. In addition, nanosecondary antibodies are very suitable for parallel detection of multiple primary antibodies, which come from different species, or even different subclasses of the same species.
