The addition of adjuvants to vaccine formulations to enhance the immune response to vaccine antigens by enhancing antigen processing and presentation by antigen presenting cells (APCs) is a common practice for current vaccine formulations. However, since many adjuvants are composed of microbial products of natural origin or synthetic analogs thereof, they cause some adverse reactions of the body. Adjuvants can interact with a series of pattern recognition receptors (PRRs) expressed on APCs such as dendritic cells and macrophages. In some cases, excessive activation of PRR on APC may result in local and systemic toxicity, including fever. Among the total number of reported cases submitted to the vaccine adverse event reporting system from 1991 to 2001, fever was the most common adverse event, which occurred in 25.8% of the reports, followed by hypersensitivity at the injection site (15.8%) and rash (11.0%). ), edema at the injection site (10.8%) and vasodilation (10.8%).
Muramyl dipeptide (MDP) is a peptidoglycan motif present in all Gram-positive and Gram-negative bacteria. Animal studies have shown that MDP has a wide range of immunomodulatory activities, including enhanced antibody production, increased cell-mediated immunity, increased non-specific immunity to bacteria and increased release of cytokines. It was found that MDP can interact with cytoplasmic PRR NOD2 (nucleotide-binding oligomerization domain 2) to transmit cellular signals, while NOD2 is highly expressed in APC, which is a necessary of humoral response to MDP adjuvant antigen. Since CFA is toxic to human, and MDP can replace the activity of the entire killed mycobacteria in complete Freund’s adjuvant (CFA), the vaccine field is working to develop a safer adjuvant based on MDP. Among them, MDP-derived moradyl ester, threonyl-MDP, and muramyl tripeptide were tested in clinical trials as adjuvants against HIV-1 and influenza virus vaccines. Clinical findings have found that a large amount of reactogenicity, including fever and other systemic reactions, has been observed in a proportion of vaccines and has hindered the further development of MDP adjuvants for human vaccines.
Mechanism of Causing Fever
In order to better develop MDP-based adjuvants, it is necessary to understand the mechanism of MDP-induced fever. When microbial products activate PRR, macrophages produce pyrogenic cytokines such as interleukin 1β (IL-1β), IL-6 and tumor necrosis factor-α. The pyrogenic cytokines released by macrophages are transported by blood flow to the hypothalamic anterior ventricle, where the release of the pyrogenic lipid mediator-prostaglandin E is induced. In addition to being produced in the central brain, PGE 2 can also be produced by macrophages in surrounding tissues, including liver Kupffer cells. Studies have shown that locally produced PGE2 can transmit a fever signal by binding to the PGE2 receptor.
The key role of PGE 2 in tissue inflammatory response has been well established: PGE 2 promotes vascular permeability and promotes the flow of neutrophils, macrophages and mast cells from the bloodstream, causing swelling and edema infection at this site. It also stimulates the sensory nerves to increase the pain response and promote fever. Studies of human monocytes activated by TLR agonists suggest that circulating monocytes may be a major source of PGE2.
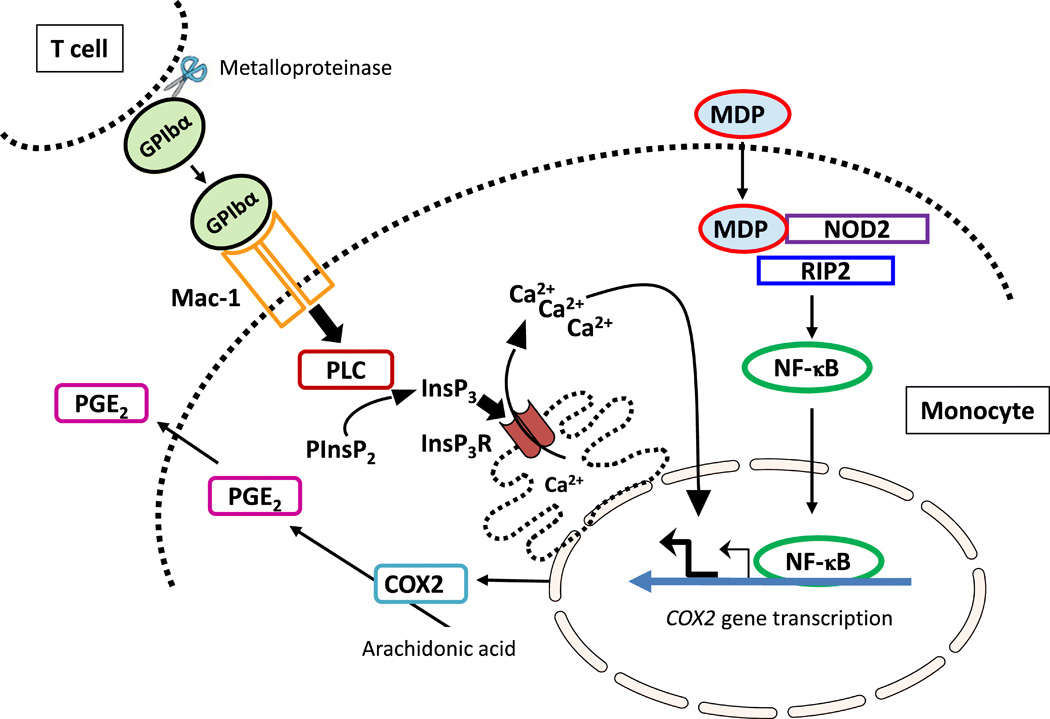
In addition, the study also found that the T cell-derived soluble glycoprotein GPIbα activates the PLC/InsP 3 / InsP 3 R pathway by triggering Mac-1 in monocytes and induces calcium release from the endoplasmic reticulum. An increase in cytosolic calcium provides a second signal that is critical for the increase in COX2 transcription in MDP-activated monocytes and subsequent PGE2 production.This is also the main cause of MDP-induced fever.
