The complement system is considered to be a biological effector system composed of a group of thermolabile enzymatically active globulins on the surface of serum, tissue fluid and cell membrane. The membrane attack complex is finally formed by a complex enzymatic cascade reaction, which realizes the lysis and destruction of target cells to protect the host body. The complement system has three intersecting pathways: the classical pathway (CL), the alternative pathway (AP), and the mannose-bing lectin (MBL), which are mainly involved in pathogen infection, local tissue damage, and Inflammation and allergy play a key role in immune regulation. Among them, the liver is the most important source of complement in the body, and the resulting complement is called systemic complement, which is mainly responsible for the complement immune regulation of serum; under local infection or injury conditions, the body tissue cells will also produce complement, called local complement. These two types of complement play a role in adhering cells, neutralizing viruses, degrading immune complexes, and regulating adaptive immunity. Therefore, complement is also regarded as a bridge connecting innate and adaptive immunity.
The complement system is an important part of the body’s innate immunity. It consists of more than 50 soluble proteins and membrane-bound proteins. It plays an important role in enhancing the humoral immune response and other life activities. Complement receptors are a class of membrane proteins that are mainly expressed on the surface of immune cell membranes. They combine with activated complement components to enhance the recruitment of leukocytes to sites of inflammation, promote phagocytosis to clear pathogenic microorganisms, and clear immune complexes produced at sites of infection or in the blood system and so on. Among them, the complement receptor 3 and the complement component 5 receptor have received much attention.
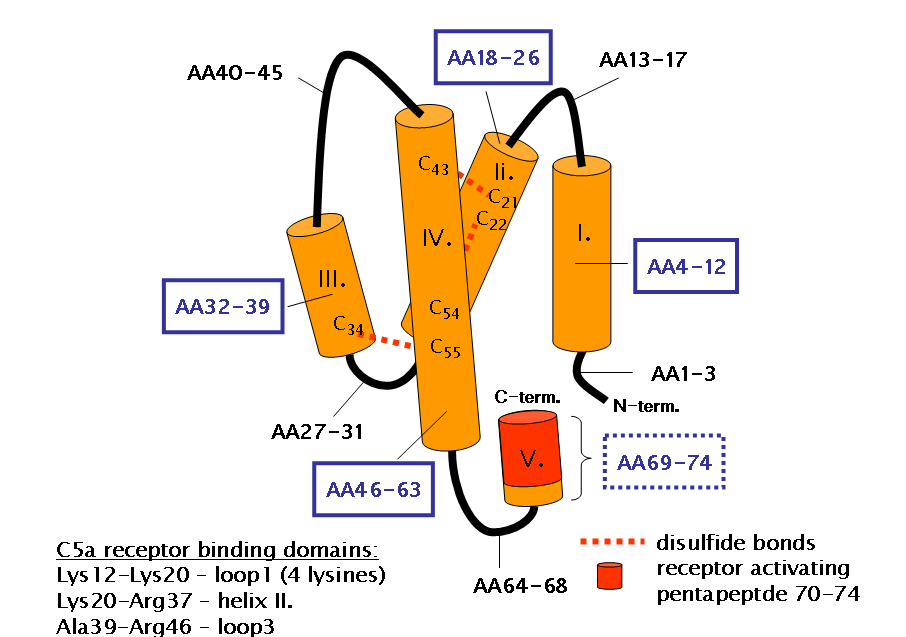
C5aR is a membrane glycoprotein with a molecular weight of 42 kda, belonging to a seven-transmembrane G protein-coupled receptor with high affinity for C5a and C5a-desarg.
Human CD4+ T cells have two C5 active components, C5a and C5a-desArg, and two C5a receptors, C5aR2 (GPR77 C5L2) and C5aR1 (CD88). Among them, C5aR1 is mainly distributed in the plasma membrane, while C5aR2 exists in the cell and plasma membrane. Studies have confirmed that cathepsin D (CTSD) can cleave C5 to generate the active component C5a, while cathepsin G (CTSG) can enzymatically degrade C5aR1 and inactivate it. In the resting state, human CD4+ T cells store a lower content of C5 and its active component C5a, while C5a receptors are dominated by C5aR2. When CD4+ T cells were stimulated by the outside world, the intracellular C5a content increased, and the C5aR receptor also turned to C5aR1. Intracellular C5a and C5aR1 are transported to the cell membrane and combined with each other, on the one hand, they can induce mitochondria to produce ROS, and on the other hand, they can promote the assembly of NLRP3 inflammasome to activate the autocrine of il-1β, and finally induce the production of interferon-γ. When the stimulation is terminated, the expression level of C5aR2 is restored and it forms a dimer with C5aR1, which can not only negatively regulate the above-mentioned intracellular signaling pathway mediated by C5aR1, but also inhibit the extracellular ERK1/2 signaling pathway mediated by C5aR1. The expression of interferon-γ in T cells decreased and the expression of il-10 increased, which promoted the rapid recovery of cells from the activated state to the resting state.
