At present, the outbreak of COVID-19 has spread to more than 200 countries and regions around the world, and the number of confirmed cases has exceeded 6 million. The world is racing to develop the Novel Coronavirus vaccine. Dozens of candidate vaccines are undergoing pre-clinical evaluation, but they also encounter difficulties. In the eyes of top scientists, the number of glycosylation sites, the number of “camouflage props” of the novel coronavirus, is twice that of HIV, and thus it is easier to deceive the human immune system to avoid being killed by antibodies, which has also become one of the bottlenecks of the current vaccine research.
Glycosylation is a widespread protein post-translational modification that is complex and variable in structure and plays an important role in cells and organisms. More than 50% of the proteins in the organism are glycosylated, including nucleoporin, chromatin protein, RNA polymerase II, protein translation regulators, transcription factors, etc. Their changes are involved in various life activities, such as cell recognition, cell differentiation, cell development, signal transduction and immune response. They are also related to occurrence and development of various diseases, such as tumors, neurodegenerative diseases, cardiovascular diseases, metabolic diseases, immune diseases and infectious diseases. Similarly, virus replication and invasion of the host are closely related to the glycosylation modification of its own structural proteins.
Comparing with the glycosylation of the envelopes of several common viruses, there are 8 to 15 glycosylation sites for Ebola virus, 5 to 11 for IAV (influenza virus), and 4 to 11 for HCV (hepatitis C virus), and 20 to 30 for HIV (AIDS virus). It has been found that the glycosylation site of SARS-CoV-2 is at least twice that of HIV.
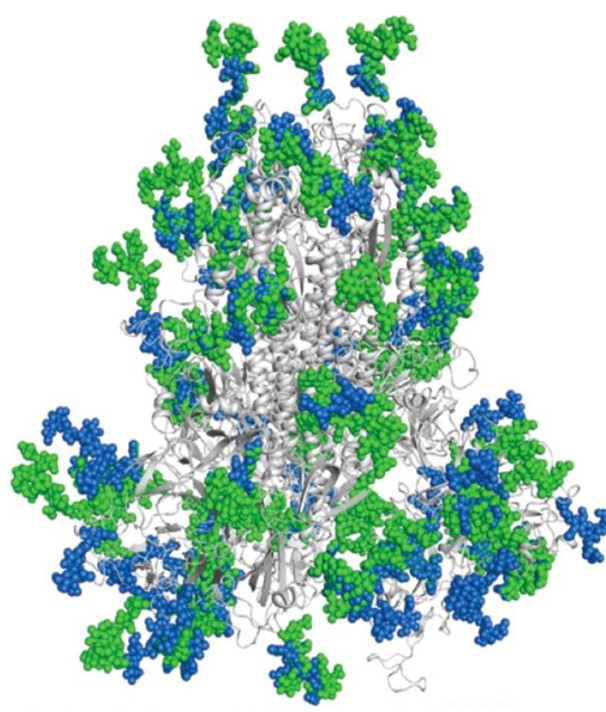
The figure below shows the predicted glycosylation sites on the viral protein shell with green the SARS-CoV-2 glycosylation site, and blue the SARS-CoV glycosylation site. Highly glycosylated sites phenomenon can be observed.
Fig 1. Predicted Glycan shield (spheres) of SARS-CoV-2 and SARS-CoV Spike Glycoproteins.
Source: DOI: 10.1080 / 22221751.2020.1739565
Professor Raymond Dwek, the founding director of the Oxford Glycobiology Institute at Oxford University, joined the online international discussion at the invitation of the World Association of Scientists (WLA). He said that this highly glycosylated sites phenomenon is bringing great difficulty to vaccine research and development. Scientists represented by Professor Dwek first discovered the glycosylation phenomenon in the virus protein shell. The new study found that SARS-CoV-2, as a highly glycosylated spherical particle, has at least 66 glycosylation sites and is more prone to mutation. This virus has many similarities with SARS-CoV in 2003. Studies have shown that of the 69 glycosylation sites of SARS-CoV, 54 are similar or identical to SARS-CoV-2.
“In a simple analogy, glycosylation sites are like camouflage props.” The vaccine is actually a “hollowed out” harmless virus so that the body’s immune system can recognize it and form antibodies endogenously. Professor Dwek said that the vaccine should evoke an immune response to attack the virus, but when the virus really invades the body, these highly camouflaged external sites can deceive the immune system’s detection and help the virus survive.
For such a global public health issue, researchers have been searching for solutions with great effort. Recently, many articles about S protein glycosylation sites and sugar chain types of the novel coronavirus have been published in various platforms and journals such as bioRxiv, Science, Cell, etc. These research papers have aroused widespread concern in the field of basic scientific research and the pharmaceutical industry.