Introduction
Malaria is caused by infection with Plasmodium parasites. Plasmodium belongs to unicellular eukaryote, and the Plasmodium that infects human body mainly comprises 5 kinds: Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale and Plasmodium knowlesi. Among them, Plasmodium falciparum and Plasmodium vivax are the most widespread in the global epidemic area, and Plasmodium falciparum is the most important species that causes the death of malaria patients. The life cycle of Plasmodium is complex and mainly includes three stages: infrared stage, red inner stage and mosquito stage. Among them, both the red inner stage and the infrared stage of Plasmodium carry out asexual development and reproduction in the human body, while the mosquito stage carries out sexual reproduction and spore proliferation and development in Anopheles mosquitoes. In order to control and eliminate malaria, corresponding candidate vaccines are mainly designed for the three stages of the life cycle of Plasmodium, in order to block the life cycle of Plasmodium from multiple links. The infrared phase is the initial stage of malaria parasite infection, and it is also the main period leading to the recurrence of malaria. Blocking the development of malaria parasites in the infrared phase can control the infection and resurgence of malaria parasites from the source. Therefore, the infrared phase vaccine is also called malaria preventive; red-phase vaccines mainly reduce clinical morbidity and mortality, so they are also called malaria therapeutic vaccines; mosquito-phase vaccines mainly block the transmission of malaria parasites, so they are also called transmission-blocking vaccines.
According to the official website of clinical trials of the National Institutes of Health, in the past 20 years, about 10 candidate malaria vaccine applications have entered clinical trials every year. Among them, the subunit vaccines of the infrared stage, the sporozoite attenuated Plasmodium vaccines, and the transmission-blocking candidate vaccines targeting the mosquito stage are the majority, while the red-stage malaria candidate vaccines registered for clinical trials have shown a significant downward trend in the past 20 years , it can be seen that the development of malaria vaccines has gradually shifted from therapeutic to prevention and blocking transmission, in order to achieve the ultimate goal of controlling and eliminating malaria on a global scale. Plasmodium vivax is not only widespread in Southeast Asia, but also the main cause of malaria recurrence, which is one of the biggest obstacles to malaria elimination. Fewer vaccine candidates against P. vivax than P. falciparum have entered clinical trials. The results of the controllable human infection with Plasmodium falciparum showed that the attenuated sporozoite vaccine against Plasmodium falciparum can provide 80% to 100% protection efficiency, but the protection efficiency dropped to below 60% after one year, and the protection efficiency of the field test efficiency rarely exceeds 50%.
Key Issues Facing Malaria Vaccine Development
It is well known that the development of an effective malaria vaccine is the most effective means for the ultimate control and elimination of malaria. Although some breakthroughs have been made in the development of malaria vaccines, there is still a long way to go before the successful development of effective malaria vaccines. Therefore, it is necessary to sort out the main technical and theoretical bottlenecks faced by the development of effective malaria vaccines from the perspective of the unique biology and immunology of Plasmodium. After combing, it was found that there are three main problems in the development of malaria vaccines: 1. The unique biological characteristics of malaria parasites; 2. The protective immune mechanism of malaria vaccines is not yet clear; 3. There are multiple immune suppression and escape strategies in malaria parasites.
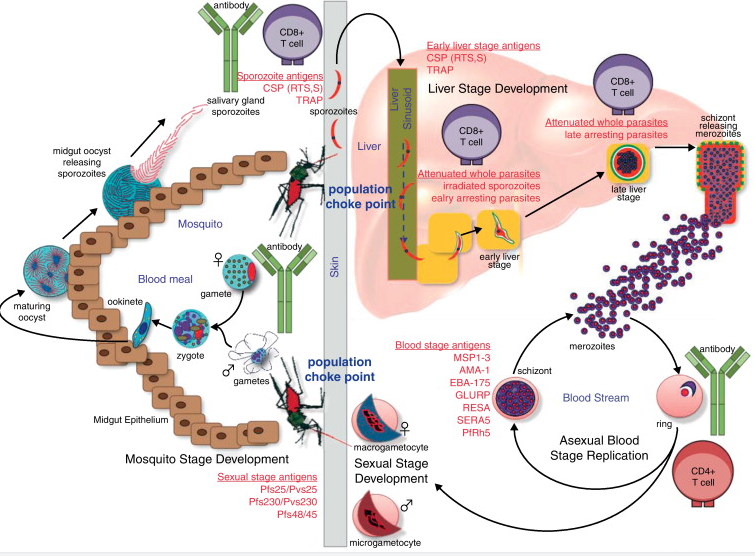
The unique biological characteristics of Plasmodium
Plasmodium is a complex organism with multiple phases
Plasmodium is a single-celled eukaryote, and its complexity is far more than that of bacteria and viruses. The results of Plasmodium genome sequencing predict that it contains about 5 300 proteins. Therefore, the immune response spectrum induced by existing subunit malaria vaccines based on one or several protective antigens is relatively narrow, and theoretically it is difficult to induce good protective immunity. In addition, unlike bacteria and viruses, Plasmodium not only has the ability to proliferate at certain stages, but more importantly, it also has the characteristics of development. The infrared stage, red inner stage and mosquito stage of Plasmodium, each stage contains multiple stages; the antigens of Plasmodium in each stage are quite different, and there is obvious stage specificity. It can be seen that a candidate vaccine against a certain stage of Plasmodium generally cannot play an effective role in other stages.
Characteristics of Plasmodium Invasion of Host Cells
Bacteria and viruses usually only need a single receptor/ligand to successfully invade host cells. However, Plasmodium invasion of host cells, including sporozoites invading liver cells and merozoites invading erythrocytes, is a multi-step, sequential and tightly regulated process. It goes through four steps: initial adhesion, localization and tight adhesion, tight junction formation, and invasion of red blood cells. Each step is mediated by different paired molecules, and the localization and tight adhesion stages can be mediated by multiple paired molecules. Adhesion mediated by different family proteins can compensate each other, that is to say, blocking one of the pathways does not significantly inhibit the invasion of Plasmodium. Under the action of the body’s immune selection pressure, these invasion-related proteins often show high polymorphism among different geographical strains. In addition, the entire invasion process is very short, lasting 30-60s. Therefore, to a certain extent, it can explain why the current erythrophase subunit vaccines that induce antibodies against these human invasion proteins cannot induce ideal protective immunity in the body. Relatively speaking, the design concept of a whole erythrocytic vaccine that induces CD4+ T cell responses against conserved intracellular antigens of Plasmodium is more promising.
Malaria Vaccine’s Mechanism of Protective Immunity Unknown
Elucidating the mechanism of host immune protection against Plasmodium is a prerequisite for the successful development of malaria vaccines. Through the continuous efforts of scientists around the world, positive breakthroughs have been made in the research on the mechanism of host immune protection against Plasmodium, but there are still many unknown problems to be solved. For example, patients in malaria-endemic areas usually cannot obtain an effective anti-sporozoite immune response and can be recurrently infected with sporozoites. However, irradiation or genetically attenuated sporozoite immunization can induce the host to produce complete immune protection against sporozoites; since mature erythrocytes do not express major histocompatibility complex class I molecules, it has long been believed that CD8+ T cells It is considered unable to recognize and kill Plasmodium erythrostages. However, recent studies have confirmed that activated CD8+ T cells in vivax patients can recognize and target Plasmodium parasites in reticulocytes through cytotoxicity; mice immunized with inactivated erythroid-stage whole-worm vaccine can not only resist the attack of erythrocytic-stage Plasmodium, but also the immune effector molecules induced by it can effectively inhibit the development of mosquito-stage Plasmodium.
Immune Evasion Mechanism of Plasmodium
At present, clinical trials of controlled human infection with Plasmodium falciparum have found that the genetically attenuated sporozoite candidate vaccine (GAS) can indeed induce good protective immunity in volunteers without a history of Plasmodium infection. However, field trials found that GAS did not induce ideal protective immunity in volunteers in malaria-endemic areas. The main reasons are as follows: 1. The protective immunity of attenuated sporozoite vaccines has species specificity. The Plasmodium falciparum challenge strain used in the experiment of controllable human infection with Plasmodium falciparum is from the same source as the vaccine strain, but the Plasmodium strains in malaria endemic areas are very complicated, and there are differences between them and the vaccine-derived strains. 2. The nutritional status of volunteers in malaria-endemic areas is relatively poor, usually accompanied by helminth infection. It has been reported that helminth infection can inhibit the protective immunity induced by malaria vaccine in immunized mice. 3. Volunteers in malaria-endemic areas have been more or less infected with Plasmodium, which affects the generation and maintenance of protective immunity.
