Inflammation is an important defense response of the body that can control and clear various endogenous or exogenous damaging factors acting on the body, and also has a role in repairing damage. However, excessive or persistent inflammation can also cause damage to tissues and organs. Calprotectin, named for its antibacterial function, is a heterodimer composed of S100A8 protein (also known as myeloid-related protein 8, calgranulin A) and S100A9 protein (also known as myeloid-related protein 14, calgranulin B), which are abundant in important inflammatory cells such as monocytes and neutrophils, and play an important role in the inflammatory process. Therefore, calprotectin has potential for diagnosis and even treatment of inflammation-related diseases, and is worth further study.
Functions of Calprotectin
Intracellular Functions of Calprotectin
Inside the cell, calprotectin interacts with cytoskeletal components such as keratin, actin, and profilin in a calcium-dependent manner, and promotes microtubule polymerization and regulates microtubule reorganization through the p38 mitogen-activated protein kinase pathway, thereby playing a role in the migration of phagocytic cells. At the same time, calprotectin can chelate transition metal ions such as manganese and zinc to prevent invading microorganisms from absorbing and utilizing them, thereby exerting an antibacterial effect. In addition, calprotectin has been shown to bind to unsaturated fatty acids such as arachidonic acid that are involved in the generation of prostaglandins and leukotrienes, and participate in their uptake and transport.
The Extracellular Functions of Calprotectin
Calprotectin is released into the extracellular space during tissue injury, cell death, and inflammatory conditions. At this time, it mainly plays a role as a damage-associated molecular pattern molecule, participating in the release of inflammatory mediators and migration of inflammatory cells through recognition by corresponding receptors. Currently known receptors for calprotectin include Toll-like receptor 4 (TLR4), advanced glycation end-product receptor, and extracellular matrix metalloproteinase inducer. TLR4 is an important pattern recognition receptor in the human body. After binding to its ligand, it can stimulate a cascade reaction through myeloid differentiation factor 88, leading to the activation of mitogen-activated protein kinase and nuclear transcription factor-κB pathways, thereby initiating an inflammatory response and causing tissue damage. The binding of advanced glycation end-product receptor to its ligand can trigger multiple downstream signaling pathways, including mitogen-activated protein kinase and nuclear transcription factor-κB, which play important roles in the inflammatory response. Extracellular matrix metalloproteinase inducer can induce the production of several matrix metalloproteinases, participating in the inflammatory response and processes such as cell proliferation, differentiation, migration, and apoptosis.
The Clinical Significance of Calprotectin
Calprotectin and Infection
Infection is the most important cause of inflammation, and various pathogens activate inflammatory cells to migrate to the site of infection and release various inflammatory mediators, including calprotectin, to regulate the progression of inflammation. At the same time, calprotectin can play an antibacterial role by binding to metal ions and physically interacting with pathogens. Studies have found that the level of calprotectin in the blood of patients with bacterial infections is significantly increased and positively correlated with the severity of sepsis. The plasma calprotectin level has high specificity and sensitivity for diagnosing and predicting infections. Therefore, some studies have suggested that calprotectin is a reliable biomarker for diagnosing bacterial sepsis. Interestingly, the regulation of infection-related inflammation by calprotectin is likely to be bidirectional. On the one hand, it enhances the inflammatory response and can lead to disease deterioration. On the other hand, it can also limit excessive inflammation to some extent.
Calprotectin and Immune-Related Diseases
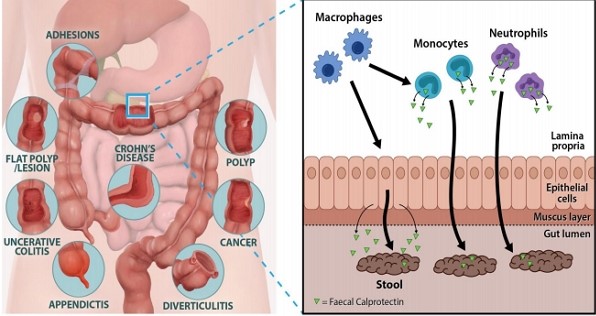
Figure 1. The role of fecal calprotectin in the gut lumen.
Abnormal immune status can damage cells and tissues, leading to inflammation. Calprotectin is expressed at increased levels in many diseases caused by immune system dysfunction. In rheumatoid arthritis, Calprotectin is produced by macrophages, polymorphonuclear cells, synovial fibroblasts, and chondrocytes, and are the most upregulated proteins in synovial tissue and synovial fluid. Although it has low sensitivity, it shows good specificity between rheumatoid arthritis patients and healthy control groups. Its concentration is closely related to disease activity, severity, and future bone erosion. In psoriatic arthritis, Calprotectin is mainly produced by mononuclear cells and neutrophils infiltrating synovial tissue. The increase in serum calcium-binding protein levels can reflect systemic inflammation, especially synovitis, and is a sensitive indicator of treatment response. Calprotectin expressed in keratinocytes promote the production of inflammatory factors and participate in the formation of psoriatic skin lesions. It can dynamically reflect the severity of skin lesions in patients, but there is no clear correlation with the level of Calprotectin in serum. In systemic lupus erythematosus, Calprotectin can be produced by mononuclear cells, polymorphonuclear cells, and cytoplasmic dendritic cells stimulated by immune complexes. They are upregulated in the kidneys and skin and may amplify and maintain inflammation. It is considered one of the indicators for monitoring disease activity and is correlated with positive anti-double-stranded DNA antibodies, arthritis, and glomerulonephritis, and may even be an effective therapeutic target. In the serum, saliva, and salivary gland tissue of patients with Sjogren’s syndrome, the concentration of Calprotectin is also significantly increased. In vitro experiments have found that Calprotectin can promote the secretion of pro-inflammatory cytokines such as interleukin-1β, interleukin-6, and tumor necrosis factor-α by peripheral blood mononuclear cells, and these cytokines can also stimulate the production of Calprotectin by peripheral blood mononuclear cells in patients.
Calprotectin and Metabolic Diseases
In the realm of metabolic diseases, including obesity and diabetes, a chronic state of low-intensity systemic inflammation prevails. Meanwhile, gout may induce acute inflammatory attacks. Calprotectin, a calcium-binding protein, exhibits a crucial function in the development of these maladies.
Obese patients demonstrate a significant rise in the circulating concentration of calprotectin and its expression in visceral adipose tissue. Furthermore, the circulation of calprotectin exhibits a positive correlation with plasma high-sensitivity C-reactive protein concentration. Hence, calprotectin may serve as a chemotactic factor, leading to macrophage recruitment into visceral adipose tissue, thereby propagating inflammation and related obesity-associated complications. As such, calcium-binding protein also constitutes a diabetes biomarker. In individuals with impaired glucose tolerance and type 2 diabetes, the concentration of this protein in their blood circulation significantly increases, coinciding with insulin resistance.
Degenerative diseases, marked by complex physiological and pathological factors, represent chronic ailments related to age and inflammation. Changes in messenger RNA abundance for S100A8 and S100A9 proteins are a conspicuous trait of mammalian tissue aging, involving diverse cell types, including the central nervous system. Consequently, calprotectin likely demonstrates an association with chronic inflammation during the aging process.