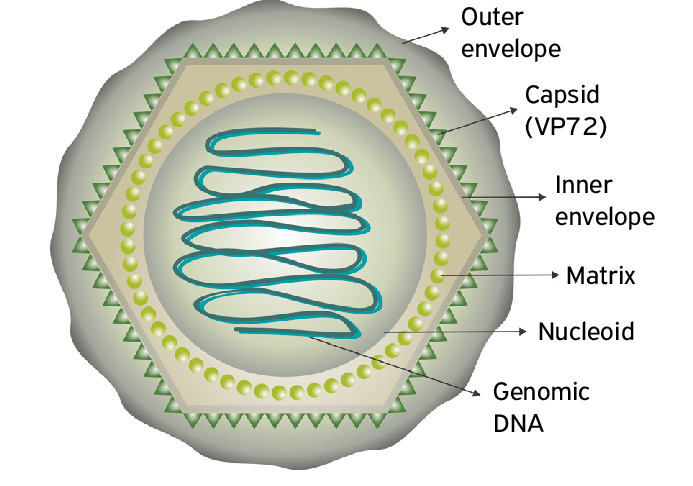
African swine fever (ASF) is a contagious infectious disease caused by African swine fever virus (ASFV) that spreads only among domestic pigs and wild boars, with a rapid onset, short course of disease, and a fatality rate of 100%. This article summarizes the latest progress in the detection technology of ASF, in order to provide a reference for the efficient diagnosis of ASF. ASFV isolation and porcine erythrocyte adsorption assay ASFV isolation combined with porcine erythrocyte adsorption assay (HAD) is one of the gold standards for detection recommended by OIE, and a positive result of the HAD test is recognized as a decisive factor in the diagnosis of ASF infection. However, due to the high technical standard requirements of primary cultured cells for the detection process, this technique is prone to false negatives during the operation, and the detection reaction takes a long time. Currently, it is usually used as an auxiliary diagnosis for other detection methods. Molecular biology-based detection methods The ASFV genome is about 170-194 kb, encoding more than 150 potential genes. The ASF molecular biology detection method is mainly based on the conserved and specific gene sequence of ASFV, and analyzes and detects the target sequence fragment through specific gene amplification. Molecular biological detection methods are favored by the detection personnel because of their accurate and efficient characteristics. Commonly used methods are: polymerase chain reaction (PCR), quantitative real-time PCR (qRT-PCR), droplet digital PCR, isothermal amplification techniques (LAMP, CPA, and RPA), CRISPR isothermal amplification techniques, and multiplex PCR (mPCR).
Serology-Based Detection Methods
Based on its advantages of simplicity, relatively low cost, and low requirements for special equipment, serological diagnostic methods are currently one of the commonly used diagnostic testing methods for ASFV. Serological detection of ASFV includes antigen detection and antibody detection, but the method of antigen detection has stricter requirements on detection conditions. Since there is currently no commercialized vaccine for ASFV, infected individuals cannot completely neutralize antibodies after infection with ASFV. Therefore, a positive test result for ASFV antibodies can directly indicate virus infection. However, some affected individuals also The onset is very rapid, and the patient died without producing antibodies. Therefore, antibody-based detection methods generally need to be combined with other detection methods for diagnosis.
Antibody Detection Method
Enzyme-linked immunosorbent assay (ELISA)
ELISA is the preferred serological detection method for ASFV recommended by OIE. Its principle is based on the indirect detection method established by the antigenic structural protein of ASFV as coating antigen. In recent years, a variety of different antigen-coated assays have been developed.
Western Blotting
Western blotting mainly uses the specific reaction of antigen-antibody to detect the target protein from the protein mixture, so as to quantitatively or qualitatively determine the existence of the target protein in the test sample, this technology integrates serological technology Compared with protein electrophoresis analysis method, it has the characteristics of high sensitivity.
Colloidal Gold Immunochromatography (GICA)
Compared with ELISA, PCR or qRT-PCR technology recommended by OIE, GICA technology has the advantage of being flexible and not limited by detection instruments, and is very suitable for rapid on-site screening. Recently, cross-primer amplification-diagnostic test strips combined with immunochromatographic strips and immunogold test strips have been established by researches and have the characteristics of rapid diagnosis, strong sensitivity and accurate results, and their trace detection effect is also very obvious.
Antigen Detection Method
Fluorescent Antibody Assay (FAT)
FAT is one of the commonly used antigen detection methods, and it is also the ASFV detection technology recommended by OIE. Its principle is to realize the identification of the corresponding antigen in the sample to be tested based on the fluorescent substance-labeled antibody. This technology has the advantages of rapidity, high sensitivity and strong specificity. An indirect ELISA developed by researchers using multiplex fluorescent bead immunoassays has high specificity for the detection of low-virulence ASFV isolates in ASFV-endemic areas.
Lateral Flow Immunochromatographic Analysis (LFIA)
LFIA is a solid-phase membrane immunoassay technology that combines immunolabeling technology and chromatographic technology. In recent years, the LFIA detection method based on the ASFV p72 gene has demonstrated high specificity, rapidity, and easy interpretation of detection results.
Double Antibody Sandwich ELISA (ELISA-double sandwichmethod)
The technical characteristics of double-antibody sandwich ELISA are that after two-step antigen or antibody recognition, the color reaction is expanded by the enzyme-labeled indirect antigen or antibody catalyzed substrate, and qualitative or quantitative analysis is carried out according to the depth of color.
