Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) belongs to the Arteriviridae family and belongs to the Arterivirus. PRRSV can be divided into type I (PRRSV type I) and type II (PRRSV type II). The main clinical symptoms are abortion, stillbirth and mummified fetus of pregnant sows, often accompanied by symptoms such as anorexia and fever.
PRRSV Genome Structure
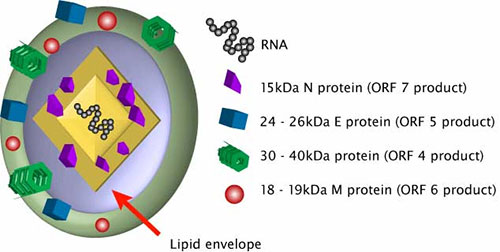
The PRRSV genome encodes 11 open reading frames (ORFs). PRRSV has 8 structural proteins, namely GP2a, GP3, GP4, GP5, membrane (M) protein, envelope (E) protein and ORF5a protein. The immunogenicity of various proteins varies. According to the latest research, GP3, M and N have the highest immunogenicity level; GP2a, E, GP4 and GP5 have lower immunogenicity; ORF5a protein is the lowest.
PRRSV Structural Protein Function
GP2a Protein
GP2a and GP4 are key viral attachment proteins that mediate viral entry into susceptible host cells by interacting with CD163, because CD163 mediates infection of PRRSV non-permissive cell lines, and PRRSV infection of Marc-145 cells can be achieved with antibodies against CD163 by block in a dose-dependent manner. And PRRSV GP2a and GP4 can induce neutralizing antibodies against PRRSV. It can be selected as a candidate antigen for new PRRSV vaccines. GP2a is essential for the production of infectious progeny. Studies have shown that the regions of amino acid positions 41–55 and 121–135 in type II PRRSV GP2a are B cell epitopes, both epitopes are conserved, and antibodies to both epitopes can reduce the virus in porcine alveolar macrophages. Indicating that GP2a can induce neutralizing antibodies against PRRSV.
GP3, GP4 Proteins
Glycoprotein 3 (GP3), a highly glycosylated membrane protein, is the protective antigen and minor structural protein of porcine reproductive and respiratory syndrome virus (PRRSV) and plays a critical role in viral assembly and infection effect. The overlapping regions of GP3 and GP4 are hypervariable, and deletions at the 3′ end of PRRSV GP3 usually result in changes in the lengths of GP3 and GP4 in PRRSV type I, and deletions are commonly found in PRRSV type VI isolates. GP4 is a minor, highly glycosylated structural protein consisting of 187 and 183 amino acids in type I and type II PRRSV, respectively. In addition, the recombinant GP4 protein using E. coli preparation and purification system can effectively recognize PRRSV positive serum, phage has strong specificity for PRRSV, and specific phage can also be used to distinguish PRRSV from other viruses.
GP5 Protein, M Protein
GP5 and M are the major membrane proteins of PRRSV, drivers of viral budding and targets of antibodies. In terms of sequence conservation, GP5 is the protein with the most variation in PRRSV, especially its N-terminal extracellular domain, so GP5 is often used in virus evolution research. GP5 forms a disulfide-linked complex including cysteine 50 with aglycosylated M protein, and this GP5/M complex is involved in viral assembly, budding, and the binding of viral particles to cell-attachment factors. The M protein may also affect reactive escape mutants against GP5 antibodies, which are resistant to broadly neutralizing antibodies due to a deletion of one amino acid in the outer domain of M. As the main glycosylated structural protein of the virus, GP5 protein has good immunogenicity and can induce the formation of antibodies with neutralizing ability higher than that induced by GP4 in vivo, and GP5 and M proteins can polymerize to form dimers. The M protein can form a heterodimer with GP5, which is essential for protein transport and progeny virion assembly and efflux. It is highly immunogenic and conserved, viral budding occurs on the membrane of the exocytosis pathway and requires GP5/M.
Envelope Protein E
The E protein is a minor structural component of the virion. The E protein of PRRSV is 70-73 amino acids in length, with a single transmembrane helical structure and ion channel activity. The E protein is not required for virion formation, but E-deleted PRRSV viruses are not infectious. In addition, the E protein has additional biological functions, interacting with host mitochondrial proteins responsible for inducing apoptosis, and is expressed at late time points in PRRSV replication, which promotes the degradation of the microtubule network in the late stages of infection. E protein also interacts with mitochondrial proteins ATP5A, inhibin, and ADP/ATP translocase, and induces apoptosis by inhibiting ATP production.
Capsid Protein N
N protein is an important polyphosphorylated protein. Can regulate the growth ability of PRRSV in Marc-145 cells. The N protein is the most abundant protein in PRRSV, which interacts with viral RNA to form nucleocapsid and participates in virion assembly. The N protein also regulates the host immune response, and it contains at least two phosphorylation modification sites. Phosphorylation of the N protein reduces viral growth and affects viral virulence.
ORF5a Protein
GP5a, also known as ORF5a protein, is a novel structural protein. Studies have shown that the ORF5a protein is closely related to viral viability and infectivity, but the current information on the genetic variation of PRRSV ORF5a gene is quite limited.
