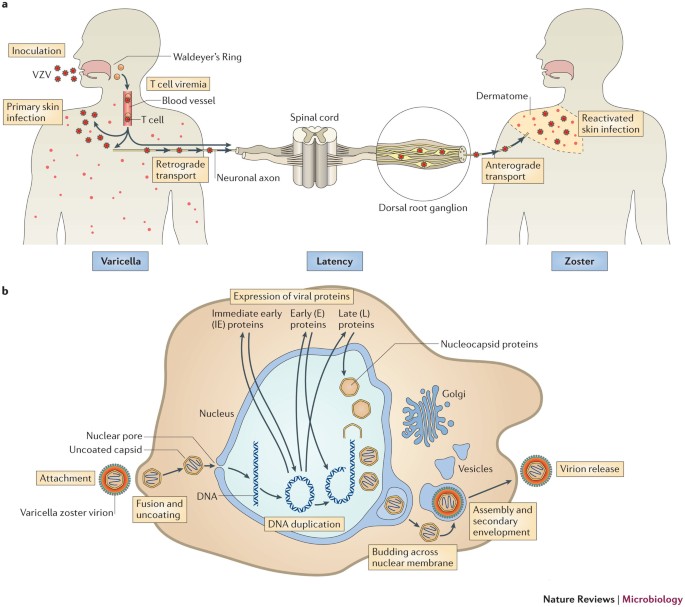
Varicella-zoster virus (VZV) is a pathogen widely present in the global population, infecting more than 90% of the world’s population. VZV primary infection often occurs in childhood, which can cause chickenpox characterized by systemic vesicular eruptions, and after infection, it can remain latent in the host’s neuronal cells. With age, immune dysfunction or other factors, the latent virus can be reactivated, spread along the peripheral nerves, and cause herpes zoster (HZ). With the popularization of vaccines, the incidence of varicella has been basically controlled, but HZ is more common in many elderly people. For people who are infected with VZV for the first time and have low immunity or VZV reactivation, they are often accompanied by complications such as meningitis, stroke, and post-HZ neuralgia, which reduce the patient’s quality of life and even endanger their lives. The pathogenesis of VZV infection is still unclear. This article focuses on studying VZV from the perspective of immunological mechanisms, in order to provide a theoretical basis for the prevention and treatment of varicella, HZ and related complications.
Figure 1. VZV life cycle and replication.
VZV Pathogenesis
Innate Immunity and Humoral Immune Response to VZV Infection
The innate immune system is the first line of defense in the host body to protect the pathogen after it enters the body. VZV infection first causes an innate immune response. Activated T lymphocytes and natural killer (NK) cells secrete interferon (IFN), which enables the host to establish a systematic antiviral system in the early stage of infection, effectively prevent and control the replication and spread of the virus, and participate in immune clearance. With the activation of VZV, the infected T cells are activated into memory T cells, which damages the body’s immune level, and eventually the virus will break through the innate immune response regulated by IFN. In the study of severe combined immune mouse models, it was found that IFN-ɑ expression was downregulated, so that VZV replication proceeded smoothly, explaining the innate immune process of VZV infection and breakthrough.
The humoral immune response can produce antiviral antibodies after the host is infected with pathogens, preventing the virus from binding to the host cell receptors, and playing a role in resisting and neutralizing the virus. VZV-specific antibodies are produced when primary infection forms chickenpox, but they cannot prevent the occurrence of HZ. The onset of HZ is related to both innate immunity and humoral immunity, especially the innate immunity.
T lymphocytes—VZV Transport and Transmission
During primary infection with VZV, the virus replicates locally in the upper respiratory tract and epithelial cells, and is transferred to T lymphocytes in the tonsils directly or through other virus-infected cells such as dendritic cells (DCs). The virus further replicates in the organs of the reticuloendothelial system such as the liver and spleen, spreads widely throughout the body, and is eventually transported to the skin and mucous membranes to produce chickenpox. At the end of the infection, VZV hides in neurons at the root of the host’s spinal cord. In the primary infection, VZV infection causes T cells to transform into memory T cells, which participate in the transport and transmission of the virus and reduce immune function. It usually takes 10 to 21 days for chickenpox to appear, which is presumably the time required for T cells to infect skin tissue. After infection, T cells increase skin homing and produce skin lesions. The SCID-hu mouse VZV infection model and the detection of VZV-infected T cells in the blood during acute infection support the role of T cells in the spread of the virus within the host.
VZV Latent Mechanism
VZV Inhibits the Expression of Molecules
The gene products encoded by VZV can downregulate the expression of MHC-I molecules in T lymphocytes, reduce the sensitivity of T lymphocytes to antigens, and reduce the immune recognition function of CD8+ T lymphocytes. Therefore, VZV has time to replicate and remain latent for a long time before being cleared by the immune system. Some researchers also believe that the nerve cells in the ganglia where VZV lurks only express a small amount or no MHC-I, thus avoiding immune recognition.
VZV Activates Cell Autophagy
Cell autophagy is one of the important defense mechanisms of the host against viruses, and is also involved in the process of the immune system clearing pathogens. However, human herpes viruses have also evolved a variety of mechanisms to avoid, destroy, and even induce the activation of cell autophagy to lurk in the human body.
VZV Reactivation Mechanism
The activation of latent VZV is the key to the onset of HZ. VZV inhibits the expression of MHC-I, MHC-II and other molecules through immune regulation, and activates T lymphocyte autophagy, making it difficult for target cells infected with VZV to be recognized by the immune system, and VZV will be reactivated. The process of VZV reactivation is closely related to the recognition of viruses by pattern recognition receptors, the interaction of T lymphocyte subsets and the release of cytokines.
| Cat (Antibody) | Product Name | Host | Application | |
| DMAB-CS24090 | Anti-VZV gE Mab, clone 1E8 | Mouse | ELISA, Neut | Inquiry |
| DMAB-CS24091 | Anti-VZV gE Mab, clone 1G6 | Mouse | ELISA, Neut | Inquiry |
| DMAB-CS24092 | Anti-VZV gE Mab, clone 1E10 | Mouse | ELISA, Neut | Inquiry |
| DMAB-CS24093 | Anti-VZV gE Mab, clone 2G7 | Mouse | ELISA, Neut | Inquiry |
| DMAB-CS24094 | Anti-VZV gE Mab, clone 1B10 | Mouse | ELISA | Inquiry |
| DMAB-CS24095 | Anti-VZV gE Mab, clone 2C9 | Mouse | ELISA | Inquiry |
| DMAB-CS24109 | Anti-VZV gE Mab (set) | Mouse | ELISA, Neut | Inquiry |
| DAGC788 | Recombinant VZV gE (a.a. 32-539) Antigen | CHO | immunoassay | Inquiry |